Oligodendrocyte Progenitors in Glial Scar: A Bet on Remyelination
- PMID: 38920654
- PMCID: PMC11202012
- DOI: 10.3390/cells13121024
Oligodendrocyte Progenitors in Glial Scar: A Bet on Remyelination
Abstract
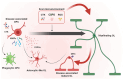
Oligodendrocyte progenitor cells (OPCs) represent a subtype of glia, giving rise to oligodendrocytes, the myelin-forming cells in the central nervous system (CNS). While OPCs are highly proliferative during development, they become relatively quiescent during adulthood, when their fate is strictly influenced by the extracellular context. In traumatic injuries and chronic neurodegenerative conditions, including those of autoimmune origin, oligodendrocytes undergo apoptosis, and demyelination starts. Adult OPCs become immediately activated; they migrate at the lesion site and proliferate to replenish the damaged area, but their efficiency is hampered by the presence of a glial scar-a barrier mainly formed by reactive astrocytes, microglia and the deposition of inhibitory extracellular matrix components. If, on the one hand, a glial scar limits the lesion spreading, it also blocks tissue regeneration. Therapeutic strategies aimed at reducing astrocyte or microglia activation and shifting them toward a neuroprotective phenotype have been proposed, whereas the role of OPCs has been largely overlooked. In this review, we have considered the glial scar from the perspective of OPCs, analysing their behaviour when lesions originate and exploring the potential therapies aimed at sustaining OPCs to efficiently differentiate and promote remyelination.
Keywords: CNS lesion; astrocytes; demyelination; glia; multiple sclerosis; oligodendrocytes; proteoglycans; remyelination; spinal cord injury.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Beck K.D., Nguyen H.X., Galvan M.D., Salazar D.L., Woodruff T.M., Anderson A.J. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: Evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 2010;133:433–447. doi: 10.1093/brain/awp322. - DOI - PMC - PubMed
-
- Dias D.O., Kalkitsas J., Kelahmetoglu Y., Estrada C.P., Tatarishvili J., Holl D., Jansson L., Banitalebi S., Amiry-Moghaddam M., Ernst A., et al. Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 2021;12:5501. doi: 10.1038/s41467-021-25585-5. - DOI - PMC - PubMed
-
- Bellver-Landete V., Bretheau F., Mailhot B., Vallières N., Lessard M., Janelle M.E., Vernoux N., Tremblay M.È., Fuehrmann T., Shoichet M.S., et al. Microglia are an essential component of the neuroprotective scar that forms after spinal cord injury. Nat. Commun. 2019;10:518. doi: 10.1038/s41467-019-08446-0. - DOI - PMC - PubMed

