Growing Role of 3D In Vitro Cell Cultures in the Study of Cellular and Molecular Mechanisms: Short Focus on Breast Cancer, Endometriosis, Liver and Infectious Diseases
- PMID: 38920683
- PMCID: PMC11201503
- DOI: 10.3390/cells13121054
Growing Role of 3D In Vitro Cell Cultures in the Study of Cellular and Molecular Mechanisms: Short Focus on Breast Cancer, Endometriosis, Liver and Infectious Diseases
Abstract
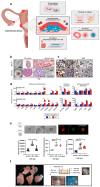
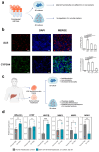
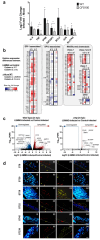
Over the past decade, the development of three-dimensional (3D) models has increased exponentially, facilitating the unravelling of fundamental and essential cellular mechanisms by which cells communicate with each other, assemble into tissues and organs and respond to biochemical and biophysical stimuli under both physiological and pathological conditions. This section presents a concise overview of the most recent updates on the significant contribution of different types of 3D cell cultures including spheroids, organoids and organ-on-chip and bio-printed tissues in advancing our understanding of cellular and molecular mechanisms. The case studies presented include the 3D cultures of breast cancer (BC), endometriosis, the liver microenvironment and infections. In BC, the establishment of 3D culture models has permitted the visualization of the role of cancer-associated fibroblasts in the delivery of exosomes, as well as the significance of the physical properties of the extracellular matrix in promoting cell proliferation and invasion. This approach has also become a valuable tool in gaining insight into general and specific mechanisms of drug resistance. Given the considerable heterogeneity of endometriosis, 3D models offer a more accurate representation of the in vivo microenvironment, thereby facilitating the identification and translation of novel targeted therapeutic strategies. The advantages provided by 3D models of the hepatic environment, in conjunction with the high throughput characterizing various platforms, have enabled the elucidation of complex molecular mechanisms underlying various threatening hepatic diseases. A limited number of 3D models for gut and skin infections have been developed. However, a more profound comprehension of the spatial and temporal interactions between microbes, the host and their environment may facilitate the advancement of in vitro, ex vivo and in vivo disease models. Additionally, it may pave the way for the development of novel therapeutic approaches in diverse research fields. The interested reader will also find concluding remarks on the challenges and prospects of using 3D cell cultures for discovering cellular and molecular mechanisms in the research areas covered in this review.
Keywords: 3D cell cultures; 3D in vitro model; bacterial infections; breast cancer; cellular and molecular mechanism; endometriosis; hepatic environment.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
- (2021, https://universitiamo.eu/en/campaigns/tumore-al-seno-sconfiggerlo-con-nanosfere-doro-intelligenti-nuove-sfide/ accessed on 19 February 2024)/Pavia University's Crowdfunding
- To the Department of Molecular Medicine of the University of Pavia under the initiative "Dipartimenti di Eccellenza (2023-2027)"./Italian Ministry of University and Research (MUR)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

