How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology
- PMID: 38920742
- PMCID: PMC11202503
- DOI: 10.3390/curroncol31060259
How to Balance Prognostic Factors in Controlled Phase II Trials: Stratified Permuted Block Randomization or Minimization? An Analysis of Clinical Trials in Digestive Oncology
Abstract
In controlled phase II trials, major prognostic factors need to be well balanced between arms. The main procedures used are SPBR (Stratified Permuted Block Randomization) and minimization. First, we provide a systematic review of the treatment allocation procedure used in gastrointestinal oncology controlled phase II trials published in 2019. Second, we performed simulations using data from six phase II studies to measure the impacts of imbalances and bias on the efficacy estimations. From the 40 articles analyzed, all mentioned randomization in both the title and abstract, the median number of patients included was 109, and 77.5% were multicenter. Of the 27 studies that reported at least one stratification variable, 10 included the center as a stratification variable, 10 used minimization, 9 used SBR, and 8 were unspecified. In real data studies, the imbalance increased with the number of centers. The total and marginal imbalances were higher with SBR than with minimization, and the difference increased with the number of centers. The efficiency estimates per arm were close to the original trial estimate in both procedures. Minimization is often used in cases of numerous centers and guarantees better similarity between arms for stratification variables for total and marginal imbalances in phase II trials.
Keywords: minimization; phase II trials; randomization.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

 ) depending on the 2 procedures: Stratified Permuted Block Randomization (left side) and minimization (right side). The characteristics of the 7 patients who were already randomized in the study are shown at the top left of the diagram. The study’s stratification variables are represented using colors and pictograms (see top right of diagram).
) depending on the 2 procedures: Stratified Permuted Block Randomization (left side) and minimization (right side). The characteristics of the 7 patients who were already randomized in the study are shown at the top left of the diagram. The study’s stratification variables are represented using colors and pictograms (see top right of diagram).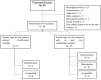

References
-
- Phase II Trials in the EORTC. The Protocol Review Committee, the Data Center, the Research and Treatment Division, and the New Drug Development Office. European Organization for Research and Treatment of Cancer. Eur. J. Cancer. 1997;33:1361–1363. - PubMed
-
- ICH Harmonised Tripartite Guideline. Statistical Principles for Clinical Trials. International Conference on Harmonisation E9 Expert Working Group. Stat. Med. 1999;18:1905–1942. - PubMed
-
- Guyatt G.H., Alexander P.E. Important Considerations for Clinical Trial Methodologies. Future Science Ltd.; London, UK: 2013. Randomization; pp. 40–60. (Future Science Book Series).
-
- Chia K.S. Randomisation: Magical Cure for Bias? Ann. Acad. Med. Singap. 2000;29:563–564. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

