Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020-2023)
- PMID: 38920845
- PMCID: PMC11203842
- DOI: 10.3390/ijns10020038
Current Status of Newborn Bloodspot Screening Worldwide 2024: A Comprehensive Review of Recent Activities (2020-2023)
Abstract
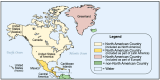
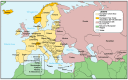
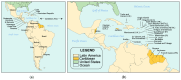
Newborn bloodspot screening (NBS) began in the early 1960s based on the work of Dr. Robert "Bob" Guthrie in Buffalo, NY, USA. His development of a screening test for phenylketonuria on blood absorbed onto a special filter paper and transported to a remote testing laboratory began it all. Expansion of NBS to large numbers of asymptomatic congenital conditions flourishes in many settings while it has not yet been realized in others. The need for NBS as an efficient and effective public health prevention strategy that contributes to lowered morbidity and mortality wherever it is sustained is well known in the medical field but not necessarily by political policy makers. Acknowledging the value of national NBS reports published in 2007, the authors collaborated to create a worldwide NBS update in 2015. In a continuing attempt to review the progress of NBS globally, and to move towards a more harmonized and equitable screening system, we have updated our 2015 report with information available at the beginning of 2024. Reports on sub-Saharan Africa and the Caribbean, missing in 2015, have been included. Tables popular in the previous report have been updated with an eye towards harmonized comparisons. To emphasize areas needing attention globally, we have used regional tables containing similar listings of conditions screened, numbers of screening laboratories, and time at which specimen collection is recommended. Discussions are limited to bloodspot screening.
Keywords: Asia–Pacific; Caribbean; Europe; Latin America; Middle East North Africa; North America; Sub-Saharan Africa; global; international data; newborn screening.
Conflict of interest statement
Marika Kase is an employee of Revvity a commercial concern active in the newborn screening field. All other authors declare no conflicts of interest.
Figures


References
-
- Alvarez D.A., Jr. Delaware’s phenylketonuria early detection and screening program. Del. Med. J. 1963;35:232–234. - PubMed
-
- Guthrie R. Blood screening for phenylketonuria. JAMA—J. Am. Med. Assoc. 1961;178:863. doi: 10.1001/jama.1961.03040470079019. - DOI
-
- Guthrie R., Susi A. A simple phenylalanine method for detecting in large populations of newborns. Pediatrics. 1963;32:338–343. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

