Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement
- PMID: 38920908
- PMCID: PMC11203111
- DOI: 10.3390/gels10060361
Dual Functionalization of Hyaluronan Dermal Fillers with Vitamin B3: Efficient Combination of Bio-Stimulation Properties with Hydrogel System Resilience Enhancement
Abstract
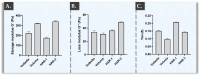
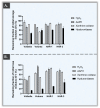
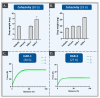
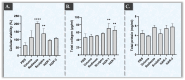
Hyaluronic acid (HA) hydrogels are commonly used for facial dermal filling and for alternative medical aesthetic purposes. High diversity exists in commercial formulations, notably for the optimization of finished product stability, functionality, and performance. Polyvalent ingredients such as calcium hydroxylapatite (CaHA) or vitamin B3 (niacinamide) are notably used as bio-stimulants to improve skin quality attributes at the administration site. The aim of the present study was to perform multi-parametric characterization of two novel cross-linked dermal filler formulas (HAR-1 "Instant Refine" and HAR-3 "Maxi Lift") for elucidation of the various functional impacts of vitamin B3 incorporation. Therefore, the HAR products were firstly comparatively characterized in terms of in vitro rheology, cohesivity, injectability, and resistance to chemical or enzymatic degradation (exposition to H2O2, AAPH, hyaluronidases, or xanthine oxidase). Then, the HAR products were assessed for cytocompatibility and in vitro bio-stimulation attributes in a primary dermal fibroblast model. The results showed enhanced resilience of the cohesive HAR hydrogels as compared to JUVÉDERM® VOLBELLA® and VOLUMA® reference products in a controlled degradation assay panel. Furthermore, significant induction of total collagen synthesis in primary dermal fibroblast cultures was recorded for HAR-1 and HAR-3, denoting intrinsic bio-stimulatory effects comparable or superior to those of the Radiesse® and Sculptra™ reference products. Original results of high translational relevance were generated herein using robust and orthogonal experimental methodologies (hydrogel degradation, functional benchmarking) and study designs. Overall, the reported results confirmed the dual functionalization role of vitamin B3 in cross-linked HA dermal fillers, with a significant enhancement of hydrogel system stability attributes and the deployment of potent bio-stimulatory capacities.
Keywords: bio-stimulation; cohesivity attributes; cross-linked dermal fillers; dermal fibroblasts; functional characterization; hyaluronic acid; hydrogel system; niacinamide; skin collagen; viscoelasticity.
Conflict of interest statement
Authors A.P., C.M., and K.L. were employed by LOUNA REGENERATIVE SA (Geneva, Switzerland) and were consultants for LOUNA AESTHETICS SAS (Poisy, France) during the course of this study. Author A.L. was employed by LAM Biotechnologies SA (Epalinges, Switzerland) and by TEC-PHARMA SA (Bercher, Switzerland) during the course of this study. The remaining authors declare no conflict of interest for this study.
Figures
References
-
- Olczyk P., Komosińska-Vassev K., Winsz-Szczotka K., Kuźnik-Trocha K., Olczyk K. Hyaluronan: Structure, metabolism, functions, and role in wound healing. Adv. Hyg. Exp. Med. 2008;62:651–659. - PubMed
-
- Saravanakumar K., Park S., Santosh S.S., Ganeshalingam A., Thiripuranathar G., Sathiyaseelan A., Vijayasarathy S., Swaminathan A., Priya V.V., Wang M.H. Application of hyaluronic acid in tissue engineering, regenerative medicine, and nanomedicine: A review. Int. J. Biol. Macromol. 2022;222:2744–2760. doi: 10.1016/j.ijbiomac.2022.10.055. - DOI - PubMed
-
- [(accessed on 9 April 2024)]; Available online: https://www.fda.gov/medical-devices/aesthetic-cosmetic-devices/dermal-fi....
LinkOut - more resources
Full Text Sources
Miscellaneous

