Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization
- PMID: 38920909
- PMCID: PMC11202472
- DOI: 10.3390/gels10060363
Antibacterial Silver Nanoparticle Containing Polydopamine Hydrogels That Enhance Re-Epithelization
Abstract
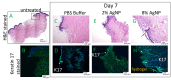
A polydopamine polyelectrolyte hydrogel was developed by ionic crosslinking dextran sulfate with a copolymer of polyethyleneimine and polydopamine. Gelation was promoted by the slow hydrolysis of glucono-δ-lactone. Within this hydrogel, silver nanoparticles were generated in situ, ranging from 25 nm to 200 nm in size. The antibacterial activity of the hydrogel was proportional to the quantity of silver nanoparticles produced, increasing as the nanoparticle count rose. The hydrogels demonstrated broad-spectrum antibacterial efficacy at concentrations up to 108 cells/mL for P. aeruginosa, K. pneumoniae, E. coli and S. aureus, the four most prevalent bacterial pathogens in chronic septic wounds. In ex vivo studies on human skin, biocompatibility was enhanced by the presence of polydopamine. Dextran sulfate is a known irritant, but formulations with polydopamine showed improved cell viability and reduced levels of the inflammatory biomarkers IL-8 and IL-1α. Silver nanoparticles can inhibit cell migration, but an ex vivo human skin study showed significant re-epithelialization in wounds treated with hydrogels containing silver nanoparticles.
Keywords: antibacterial; ionic crosslinking; polydopamine; silver nanoparticle; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Multifunctional ultrasmall AgNP hydrogel accelerates healing of S. aureus infected wounds.Acta Biomater. 2021 Jul 1;128:420-434. doi: 10.1016/j.actbio.2021.04.007. Epub 2021 Apr 20. Acta Biomater. 2021. PMID: 33857695
-
Silver nanoparticle impregnated chitosan-PEG hydrogel enhances wound healing in diabetes induced rabbits.Int J Pharm. 2019 Mar 25;559:23-36. doi: 10.1016/j.ijpharm.2019.01.019. Epub 2019 Jan 19. Int J Pharm. 2019. PMID: 30668991
-
Polydopamine Antioxidant Hydrogels for Wound Healing Applications.Gels. 2020 Oct 31;6(4):39. doi: 10.3390/gels6040039. Gels. 2020. PMID: 33142677 Free PMC article.
-
Nano-silver-incorporated biomimetic polydopamine coating on a thermoplastic polyurethane porous nanocomposite as an efficient antibacterial wound dressing.J Nanobiotechnology. 2018 Nov 12;16(1):89. doi: 10.1186/s12951-018-0416-4. J Nanobiotechnology. 2018. PMID: 30419925 Free PMC article.
-
Nanoparticle-driven self-assembling injectable hydrogels provide a multi-factorial approach for chronic wound treatment.Acta Biomater. 2021 Oct 15;134:131-143. doi: 10.1016/j.actbio.2021.07.020. Epub 2021 Jul 14. Acta Biomater. 2021. PMID: 34271166
Cited by
-
Research on the impact of polydopamine hydrogel electrodes with various doping methods on the performance of microbial fuel cells.Bioprocess Biosyst Eng. 2025 Jun;48(6):951-970. doi: 10.1007/s00449-025-03154-0. Epub 2025 Apr 19. Bioprocess Biosyst Eng. 2025. PMID: 40252082
-
Bioinspired Nanoplatforms: Polydopamine and Exosomes for Targeted Antimicrobial Therapy.Polymers (Basel). 2025 Jun 16;17(12):1670. doi: 10.3390/polym17121670. Polymers (Basel). 2025. PMID: 40574198 Free PMC article. Review.
References
-
- El Yakhlifi S., Alfieri M.-L., Arntz Y., Eredia M., Ciesielski A., Samorì P., d’Ischia M., Ball V. Oxidant-dependent antioxidant activity of polydopamine films: The chemistry-morphology interplay. Colloids Surf. A Physicochem. Eng. Asp. 2021;614:126134. doi: 10.1016/j.colsurfa.2021.126134. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

