Synthesis of Chitosan and Ferric-Ion (Fe3+)-Doped Brushite Mineral Cancellous Bone Scaffolds
- PMID: 38921188
- PMCID: PMC11202294
- DOI: 10.3390/biomimetics9060308
Synthesis of Chitosan and Ferric-Ion (Fe3+)-Doped Brushite Mineral Cancellous Bone Scaffolds
Abstract
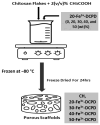
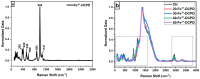
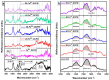
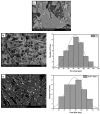
Biodegradable scaffolds are needed to repair bone defects. To promote the resorption of scaffolds, a large surface area is required to encourage neo-osteogenesis. Herein, we describe the synthesis and freeze-drying methodologies of ferric-ion (Fe3+) doped Dicalcium Phosphate Dihydrate mineral (DCPD), also known as brushite, which has been known to favour the in situ condition for osteogenesis. In this investigation, the role of chitosan during the synthesis of DCPD was explored to enhance the antimicrobial, scaffold pore distribution, and mechanical properties post freeze-drying. During the synthesis of DCPD, the calcium nitrate solution was hydrolysed with a predetermined stoichiometric concentration of ammonium phosphate. During the hydrolysis reaction, 10 (mol)% iron (Fe3+) nitrate (Fe(NO3)3) was incorporated, and the DCPD minerals were precipitated (Fe3+-DCPD). Chitosan stir-mixed with Fe3+-DCPD minerals was freeze-dried to create scaffolds. The structural, microstructural, and mechanical properties of freeze-dried materials were characterized.
Keywords: Fe3+-doped brushite (dicalcium phosphate dihydrate); bone tissue engineering; chitosan; mechanical properties; scaffold.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Dicalcium Phosphate Dihydrate Mineral Loaded Freeze-Dried Scaffolds for Potential Synthetic Bone Applications.Materials (Basel). 2022 Sep 8;15(18):6245. doi: 10.3390/ma15186245. Materials (Basel). 2022. PMID: 36143561 Free PMC article.
-
Ferric iron incorporation promotes brushite hydrolysis and enhances cadmium immobilization.Sci Total Environ. 2021 Jul 15;778:146266. doi: 10.1016/j.scitotenv.2021.146266. Epub 2021 Mar 8. Sci Total Environ. 2021. PMID: 33721635
-
Role of Aspartic and Polyaspartic Acid on the Synthesis and Hydrolysis of Brushite.J Funct Biomater. 2019 Feb 1;10(1):11. doi: 10.3390/jfb10010011. J Funct Biomater. 2019. PMID: 30717259 Free PMC article.
-
The role of brushite and octacalcium phosphate in apatite formation.Crit Rev Oral Biol Med. 1992;3(1-2):61-82. doi: 10.1177/10454411920030010601. Crit Rev Oral Biol Med. 1992. PMID: 1730071 Review.
-
Ion-doped Brushite Cements for Bone Regeneration.Acta Biomater. 2021 Mar 15;123:51-71. doi: 10.1016/j.actbio.2021.01.004. Epub 2021 Jan 14. Acta Biomater. 2021. PMID: 33454382 Review.
Cited by
-
Evaluation of the Effectiveness of Chitosan-Modified Bone Regeneration Materials: A Systematic Review.Pharmaceutics. 2025 May 18;17(5):665. doi: 10.3390/pharmaceutics17050665. Pharmaceutics. 2025. PMID: 40430955 Free PMC article. Review.
-
Chitosan Scaffolds from Crustacean and Fungal Sources: A Comparative Study for Bone-Tissue-Engineering Applications.Bioengineering (Basel). 2024 Jul 16;11(7):720. doi: 10.3390/bioengineering11070720. Bioengineering (Basel). 2024. PMID: 39061802 Free PMC article.
References
-
- Bezstarosti H., Metsemakers W.J., van Lieshout E.M.M., Voskamp L.W., Kortram K., McNally M.A., Marais L.C., Verhofstad M.H.J. Management of critical-sized bone defects in the treatment of fracture-related infection: A systematic review and pooled analysis. Arch. Orthop. Trauma Surg. 2021;141:1215–1230. doi: 10.1007/s00402-020-03525-0. - DOI - PMC - PubMed
-
- Martin R.B., Burr D.B., Sharkey N.A., Fyhrie D.P. Skeletal Tissue Mechanics. Springer; New York, NY, USA: 1998.
-
- Zárate-Kalfópulos B., Reyes-Sánchez A. Bone grafts in orthopedic surgery. Cir. Cir. 2006;74:217–222. - PubMed
LinkOut - more resources
Full Text Sources

