Histone H3 N-Terminal Lysine Acetylation Governs Fungal Growth, Conidiation, and Pathogenicity through Regulating Gene Expression in Fusarium pseudograminearum
- PMID: 38921366
- PMCID: PMC11204548
- DOI: 10.3390/jof10060379
Histone H3 N-Terminal Lysine Acetylation Governs Fungal Growth, Conidiation, and Pathogenicity through Regulating Gene Expression in Fusarium pseudograminearum
Abstract
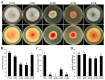
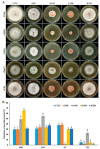
The acetylation of histone lysine residues regulates multiple life processes, including growth, conidiation, and pathogenicity in filamentous pathogenic fungi. However, the specific function of each lysine residue at the N-terminus of histone H3 in phytopathogenic fungi remains unclear. In this study, we mutated the N-terminal lysine residues of histone H3 in Fusarium pseudograminearum, the main causal agent of Fusarium crown rot of wheat in China, which also produces deoxynivalenol (DON) toxins harmful to humans and animals. Our findings reveal that all the FpH3K9R, FpH3K14R, FpH3K18R, and FpH3K23R mutants are vital for vegetative growth and conidiation. Additionally, FpH3K14 regulates the pathogen's sensitivity to various stresses and fungicides. Despite the slowed growth of the FpH3K9R and FpH3K23R mutants, their pathogenicity towards wheat stems and heads remains unchanged. However, the FpH3K9R mutant produces more DON. Furthermore, the FpH3K14R and FpH3K18R mutants exhibit significantly reduced virulence, with the FpH3K18R mutant producing minimal DON. In the FpH3K9R, FpH3K14R, FpH3K18R, and FpH3K23R mutants, there are 1863, 1400, 1688, and 1806 downregulated genes, respectively, compared to the wild type. These downregulated genes include many that are crucial for growth, conidiation, pathogenicity, and DON production, as well as some essential genes. Gene ontology (GO) enrichment analysis indicates that genes downregulated in the FpH3K14R and FpH3K18R mutants are enriched for ribosome biogenesis, rRNA processing, and rRNA metabolic process. This suggests that the translation machinery is abnormal in the FpH3K14R and FpH3K18R mutants. Overall, our findings suggest that H3 N-terminal lysine residues are involved in regulating the expression of genes with important functions and are critical for fungal development and pathogenicity.
Keywords: Fusarium crown rot; H3 N-terminal lysine residues; gene expression; histone acetylation; phytopathogen.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
FpCBS Affects a Diverse Range of Functions of Fusarium pseudograminearum Impacting Its Virulence to Wheat.Plant Cell Environ. 2025 Sep;48(9):6587-6604. doi: 10.1111/pce.15622. Epub 2025 May 23. Plant Cell Environ. 2025. PMID: 40405798
-
The Sirtuin Family FvSIRT5 and FvSIR2 Are Important for Fumonisin B Biosynthesis and Conidiation in Fusarium verticillioides.Phytopathology. 2025 Jun 22. doi: 10.1094/PHYTO-10-24-0308-R. Online ahead of print. Phytopathology. 2025. PMID: 40544456
-
Fungal Pathogens Associated with Crown and Root Rot in Wheat-Growing Areas of Northern Kyrgyzstan.J Fungi (Basel). 2023 Jan 16;9(1):124. doi: 10.3390/jof9010124. J Fungi (Basel). 2023. PMID: 36675945 Free PMC article.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
-
Antidepressants for pain management in adults with chronic pain: a network meta-analysis.Health Technol Assess. 2024 Oct;28(62):1-155. doi: 10.3310/MKRT2948. Health Technol Assess. 2024. PMID: 39367772 Free PMC article.
Cited by
-
Relationships between Wheat Development, Soil Properties, and Rhizosphere Mycobiota.Microorganisms. 2024 Jul 24;12(8):1516. doi: 10.3390/microorganisms12081516. Microorganisms. 2024. PMID: 39203359 Free PMC article.
References
Grants and funding
- ZR2021QC059/Department of Science and Technology of Shandong Province
- SKLCSRHPKF07/State Key Laboratory for Crop Stress Resistance and High-Efficiency Production of NWAFU
- 32102181/National Natural Science Foundation of China
- SDAIT-01-10/Shandong Provincial Department of Agriculture and Rural Affairs
LinkOut - more resources
Full Text Sources

