DNA Damage Checkpoints Govern Global Gene Transcription and Exhibit Species-Specific Regulation on HOF1 in Candida albicans
- PMID: 38921373
- PMCID: PMC11204775
- DOI: 10.3390/jof10060387
DNA Damage Checkpoints Govern Global Gene Transcription and Exhibit Species-Specific Regulation on HOF1 in Candida albicans
Abstract
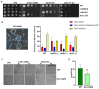
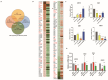
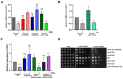
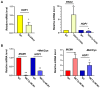
DNA damage checkpoints are essential for coordinating cell cycle arrest and gene transcription during DNA damage response. Exploring the targets of checkpoint kinases in Saccharomyces cerevisiae and other fungi has expanded our comprehension of the downstream pathways involved in DNA damage response. While the function of checkpoint kinases, specifically Rad53, is well documented in the fungal pathogen Candida albicans, their targets remain poorly understood. In this study, we explored the impact of deleting RAD53 on the global transcription profiles and observed alterations in genes associated with ribosome biogenesis, DNA replication, and cell cycle. However, the deletion of RAD53 only affected a limited number of known DNA damage-responsive genes, including MRV6 and HMX1. Unlike S. cerevisiae, the downregulation of HOF1 transcription in C. albicans under the influence of Methyl Methanesulfonate (MMS) did not depend on Dun1 but still relied on Rad53 and Rad9. In addition, the transcription factor Mcm1 was identified as a regulator of HOF1 transcription, with evidence of dynamic binding to its promoter region; however, this dynamic binding was interrupted following the deletion of RAD53. Furthermore, Rad53 was observed to directly interact with the promoter region of HOF1, thus suggesting a potential role in governing its transcription. Overall, checkpoints regulate global gene transcription in C. albicans and show species-specific regulation on HOF1; these discoveries improve our understanding of the signaling pathway related to checkpoints in this pathogen.
Keywords: Candida albicans; DNA damage response; Hof1; RNA-seq; Rad53; methyl methanesulfonate.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Hof1 plays a checkpoint-related role in MMS-induced DNA damage response in Candida albicans.Mol Biol Cell. 2020 Mar 1;31(5):348-359. doi: 10.1091/mbc.E19-06-0316. Epub 2020 Jan 15. Mol Biol Cell. 2020. PMID: 31940254 Free PMC article.
-
Transcriptional Profiling of the Candida albicans Response to the DNA Damage Agent Methyl Methanesulfonate.Int J Mol Sci. 2022 Jul 7;23(14):7555. doi: 10.3390/ijms23147555. Int J Mol Sci. 2022. PMID: 35886903 Free PMC article.
-
Characterization of Pph3-mediated dephosphorylation of Rad53 during methyl methanesulfonate-induced DNA damage repair in Candida albicans.Biochem J. 2017 Mar 23;474(7):1293-1306. doi: 10.1042/BCJ20160889. Biochem J. 2017. PMID: 28183985
-
Loss of Gst1 enhances resistance to MMS by reprogramming the transcription of DNA damage response genes in a Rad53-dependent manner in Candida albicans.Cell Commun Signal. 2024 Oct 14;22(1):495. doi: 10.1186/s12964-024-01865-7. Cell Commun Signal. 2024. PMID: 39402632 Free PMC article.
-
DDR Inc., one business, two associates.Curr Genet. 2019 Apr;65(2):445-451. doi: 10.1007/s00294-018-0908-7. Epub 2018 Nov 22. Curr Genet. 2019. PMID: 30467717 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

