Mitochondrial Protease Oct1p Regulates Mitochondrial Homeostasis and Influences Pathogenicity through Affecting Hyphal Growth and Biofilm Formation Activities in Candida albicans
- PMID: 38921377
- PMCID: PMC11204688
- DOI: 10.3390/jof10060391
Mitochondrial Protease Oct1p Regulates Mitochondrial Homeostasis and Influences Pathogenicity through Affecting Hyphal Growth and Biofilm Formation Activities in Candida albicans
Abstract
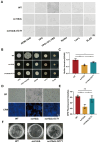
Mitochondria, as the core metabolic organelles, play a crucial role in aerobic respiration/biosynthesis in fungi. Numerous studies have demonstrated a close relationship between mitochondria and Candida albicans virulence and drug resistance. Here, we report an octapeptide-aminopeptidase located in the mitochondrial matrix named Oct1p. Its homolog in the model fungus Saccharomyces cerevisiae is one of the key proteins in maintaining mitochondrial respiration and protein stability. In this study, we utilized evolutionary tree analysis, gene knockout experiments, mitochondrial function detection, and other methods to demonstrate the impact of Oct1p on the mitochondrial function of C. albicans. Furthermore, through transcriptome analysis, real-time quantitative PCR, and morphological observation, we discovered that the absence of Oct1p results in functional abnormalities in C. albicans, affecting hyphal growth, cell adhesion, and biofilm formation. Finally, the in vivo results of the infection of Galleria mellonella larvae and vulvovaginal candidiasis in mice indicate that the loss of Oct1p led to the decreased virulence of C. albicans. In conclusion, this study provides a solid theoretical foundation for treating Candida diseases, developing new targeted drugs, and serves as a valuable reference for investigating the connection between mitochondria and virulence in other pathogenic fungi.
Keywords: Candida albicans; Oct1p; hypha formation; mitochondria; virulence.
Conflict of interest statement
We declare that we have no conflicts of interest.
Figures





Similar articles
-
Repurposing Pilocarpine Hydrochloride for Treatment of Candida albicans Infections.mSphere. 2019 Jan 23;4(1):e00689-18. doi: 10.1128/mSphere.00689-18. mSphere. 2019. PMID: 30674648 Free PMC article.
-
Pullulan nanoparticles inhibit the pathogenicity of Candida albicans by regulating hypha-related gene expression.Microbiol Spectr. 2024 Nov 14;12(12):e0104824. doi: 10.1128/spectrum.01048-24. Online ahead of print. Microbiol Spectr. 2024. PMID: 39540747 Free PMC article.
-
Unique, Diverged, and Conserved Mitochondrial Functions Influencing Candida albicans Respiration.mBio. 2019 Jun 25;10(3):e00300-19. doi: 10.1128/mBio.00300-19. mBio. 2019. PMID: 31239372 Free PMC article.
-
Suppression of hyphal formation and virulence of Candida albicans by natural and synthetic compounds.Biofouling. 2021 Jul;37(6):626-655. doi: 10.1080/08927014.2021.1948538. Epub 2021 Jul 20. Biofouling. 2021. PMID: 34284656 Review.
-
Germ tube growth of Candida albicans.Curr Top Med Mycol. 1997 Dec;8(1-2):43-55. Curr Top Med Mycol. 1997. PMID: 9504066 Review.
Cited by
-
Antifungal activity and mechanism of novel peptide Glycine max antimicrobial peptide (GmAMP) against fluconazole-resistant Candida tropicalis.PeerJ. 2025 May 20;13:e19372. doi: 10.7717/peerj.19372. eCollection 2025. PeerJ. 2025. PMID: 40416617 Free PMC article.
-
Mitochondrial anchor protein Num11 is key to pathogenicity of Candida albicans by affecting mitochondrial function and cell wall masking.Virulence. 2025 Dec;16(1):2519149. doi: 10.1080/21505594.2025.2519149. Epub 2025 Jun 18. Virulence. 2025. PMID: 40531863 Free PMC article.
References
-
- Zeng L.B., Huang Y.C., Tan J.J., Peng J., Hu N.Y., Liu Q., Cao Y.L., Zhang Y.P., Chen J.Z., Huang X.T. QCR7 affects the virulence of Candida albicans and the uptake of multiple carbon sources present in different host niches. Front. Cell. Infect. Microbiol. 2023;13:1136698. doi: 10.3389/fcimb.2023.1136698. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

