Phenylpropanoid Metabolism in Phaseolus vulgaris during Growth under Severe Drought
- PMID: 38921454
- PMCID: PMC11205357
- DOI: 10.3390/metabo14060319
Phenylpropanoid Metabolism in Phaseolus vulgaris during Growth under Severe Drought
Abstract
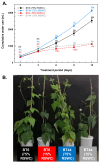
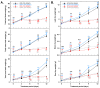
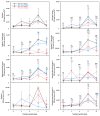
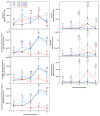
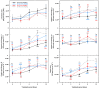
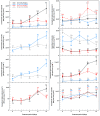
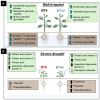
Drought limits the growth and development of Phaseolus vulgaris L. (known as common bean). Common bean plants contain various phenylpropanoids, but it is not known whether the levels of these metabolites are altered by drought. Here, BT6 and BT44, two white bean recombinant inbred lines (RILs), were cultivated under severe drought. Their respective growth and phenylpropanoid profiles were compared to those of well-irrigated plants. Both RILs accumulated much less biomass in their vegetative parts with severe drought, which was associated with more phaseollin and phaseollinisoflavan in their roots relative to well-irrigated plants. A sustained accumulation of coumestrol was evident in BT44 roots with drought. Transient alterations in the leaf profiles of various phenolic acids occurred in drought-stressed BT6 and BT44 plants, including the respective accumulation of two separate caftaric acid isomers and coutaric acid (isomer 1) relative to well-irrigated plants. A sustained rise in fertaric acid was observed in BT44 with drought stress, whereas the greater amount relative to well-watered plants was transient in BT6. Apart from kaempferol diglucoside (isomer 2), the concentrations of most leaf flavonol glycosides were not altered with drought. Overall, fine tuning of leaf and root phenylpropanoid profiles occurs in white bean plants subjected to severe drought.
Keywords: Phaseolus vulgaris; drought; flavonol glycosides; isoflavones; phenylpropanoids.
Conflict of interest statement
The authors declare no conflicts of interest. The funder had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Proteomic analysis of common bean stem under drought stress using in-gel stable isotope labeling.J Plant Physiol. 2017 Feb;209:42-50. doi: 10.1016/j.jplph.2016.10.015. Epub 2016 Dec 5. J Plant Physiol. 2017. PMID: 28013170
-
Exogenous γ-aminobutyric acid (GABA)-induced signaling events and field performance associated with mitigation of drought stress in Phaseolus vulgaris L.Plant Signal Behav. 2021 Feb 1;16(2):1853384. doi: 10.1080/15592324.2020.1853384. Epub 2020 Dec 23. Plant Signal Behav. 2021. PMID: 33356834 Free PMC article.
-
Root and shoot variation in relation to potential intermittent drought adaptation of Mesoamerican wild common bean (Phaseolus vulgaris L.).Ann Bot. 2019 Nov 27;124(6):917-932. doi: 10.1093/aob/mcy221. Ann Bot. 2019. PMID: 30596881 Free PMC article.
-
Differential proteomic analysis of drought stress response in leaves of common bean (Phaseolus vulgaris L.).J Proteomics. 2013 Jan 14;78:254-72. doi: 10.1016/j.jprot.2012.09.021. Epub 2012 Sep 29. J Proteomics. 2013. PMID: 23026550
-
Screening of Indanoyl-Type Compounds as Elicitors of Isoflavonoid Phytoalexins in Colombian Common Bean Cultivars.Molecules. 2022 May 30;27(11):3500. doi: 10.3390/molecules27113500. Molecules. 2022. PMID: 35684438 Free PMC article.
Cited by
-
Phenylpropanoids metabolism: recent insight into stress tolerance and plant development cues.Front Plant Sci. 2025 Jun 26;16:1571825. doi: 10.3389/fpls.2025.1571825. eCollection 2025. Front Plant Sci. 2025. PMID: 40641862 Free PMC article. Review.
-
Tolerance Mechanisms and Removal Efficiency of Chlorella pyrenoidosa in Treating 3-Fluorophenol Pollution.Metabolites. 2024 Aug 15;14(8):449. doi: 10.3390/metabo14080449. Metabolites. 2024. PMID: 39195545 Free PMC article.
References
-
- IPCC . “Summary for Policymakers” in Global Warming of 1.5 °C. In: Masson-Delmotte V., Zhai P., Pörtner H.-O., Roberts D., Skea J., Shukla P.R., Pirani A., Moufouma-Okia W., Péan C., Pidcock R., et al., editors. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. World Meteorological Organization; Geneva, Switzerland: 2018. pp. 3–24.
-
- Losa A., Vorster J., Cominelli E., Sparvoli F., Paolo D., Sala T., Ferrari M., Carbonaro M., Marconi S., Camilli E., et al. Drought and heat affect common bean minerals and human diet—What we know and where to go. Food Energy Secur. 2022;11:e351. doi: 10.1002/fes3.351. - DOI
-
- FAOSTAT Database Food and Agriculture Organization of the United Nations, Statistics Division, Rome. 2013. [(accessed on 24 February 2024)]. Available online: http://fenix.fao.org/faostat/beta/en/#home.
-
- Uebersax M.A., Cichy K.A., Gomez F.E., Porch T.G., Heitholt J., Osorno J.M., Kamfwa K., Snapp S.S., Bales S. Dry beans (Phaseolus vulgaris L.) as a vital component of sustainable agriculture and food security—A review. Legume Sci. 2023;5:e155. doi: 10.1002/leg3.155. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

