Nicotinamide Mononucleotide Supplementation: Understanding Metabolic Variability and Clinical Implications
- PMID: 38921475
- PMCID: PMC11205942
- DOI: 10.3390/metabo14060341
Nicotinamide Mononucleotide Supplementation: Understanding Metabolic Variability and Clinical Implications
Abstract
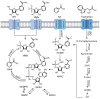
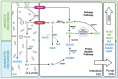
Recent years have seen a surge in research focused on NAD+ decline and potential interventions, and despite significant progress, new discoveries continue to highlight the complexity of NAD+ biology. Nicotinamide mononucleotide (NMN), a well-established NAD+ precursor, has garnered considerable interest due to its capacity to elevate NAD+ levels and induce promising health benefits in preclinical models. Clinical trials investigating NMN supplementation have yielded variable outcomes while shedding light on the intricacies of NMN metabolism and revealing the critical roles played by gut microbiota and specific cellular uptake pathways. Individual variability in factors such as lifestyle, health conditions, genetics, and gut microbiome composition likely contributes to the observed discrepancies in clinical trial results. Preliminary evidence suggests that NMN's effects may be context-dependent, varying based on a person's physiological state. Understanding these nuances is critical for definitively assessing the impact of manipulating NAD+ levels through NMN supplementation. Here, we review NMN metabolism, focusing on current knowledge, pinpointing key areas where further research is needed, and outlining future directions to advance our understanding of its potential clinical significance.
Keywords: NAD+; bioavailability; human longevity; nicotinamide mononucleotide; vitamin B3.
Conflict of interest statement
Candace Benjamin and Rebecca Crews are employees of Renue By Science The paper reflects the views of the scientists, and not the company.
Figures

Similar articles
-
The Safety and Antiaging Effects of Nicotinamide Mononucleotide in Human Clinical Trials: an Update.Adv Nutr. 2023 Nov;14(6):1416-1435. doi: 10.1016/j.advnut.2023.08.008. Epub 2023 Aug 22. Adv Nutr. 2023. PMID: 37619764 Free PMC article. Review.
-
Nicotinamide mononucleotide (NMN) as an anti-aging health product - Promises and safety concerns.J Adv Res. 2021 Aug 11;37:267-278. doi: 10.1016/j.jare.2021.08.003. eCollection 2022 Mar. J Adv Res. 2021. PMID: 35499054 Free PMC article. Review.
-
Complementary yet divergent effects of exercise and an exercise mimetic on microbiome in high-fat diet-induced obesity.Physiol Genomics. 2024 Feb 1;56(2):136-144. doi: 10.1152/physiolgenomics.00066.2023. Epub 2023 Nov 27. Physiol Genomics. 2024. PMID: 38009223
-
Host-microbiome interactions in nicotinamide mononucleotide (NMN) deamidation.FEBS Lett. 2023 Sep;597(17):2196-2220. doi: 10.1002/1873-3468.14698. Epub 2023 Aug 9. FEBS Lett. 2023. PMID: 37463842
-
The role of nicotinamide mononucleotide (NMN) in anti-aging, longevity, and its potential for treating chronic conditions.Mol Biol Rep. 2022 Oct;49(10):9737-9748. doi: 10.1007/s11033-022-07459-1. Epub 2022 Apr 20. Mol Biol Rep. 2022. PMID: 35441939 Review.
Cited by
-
Nicotinamide and Pyridoxine in Muscle Aging: Nutritional Regulation of Redox, Inflammation, and Regeneration.Antioxidants (Basel). 2025 Jul 25;14(8):911. doi: 10.3390/antiox14080911. Antioxidants (Basel). 2025. PMID: 40867810 Free PMC article. Review.
References
-
- Harden A., Young W.J. The Alcoholic Ferment of Yeast-Juice. Proc. R. Soc. Lond. B. 1906;77:405–420. doi: 10.1098/rspb.1906.0029. - DOI
-
- Melkonian E.A., Schury M.P. Biochemistry, Anaerobic Glycolysis. StatPearls Publishing; Treasure Island, FL, USA: 2023. - PubMed
-
- Leary S.C., Moyes C.D. Chapter 15—The Effects of Bioenergetic Stress and Redox Balance on the Expression of Genes Critical to Mitochondrial Function. In: Storey K.B., Storey J.M., editors. Cell and Molecular Response to Stress. Volume 1. Elsevier; Amsterdam, The Netherlands: 2000. pp. 209–229. Environmental Stressors and Gene Responses.
Publication types
LinkOut - more resources
Full Text Sources

