Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study
- PMID: 38921485
- PMCID: PMC11205401
- DOI: 10.3390/membranes14060118
Cryptotanshinone-Induced Permeabilization of Model Phospholipid Membranes: A Biophysical Study
Abstract
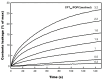
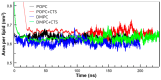
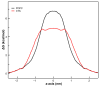
The Danshen terpenoid cryptotanshinone (CPT) is gaining enormous interest in light of its various outstanding biological activities. Among those, CPT has been shown to interact with cell membranes and, for instance, to have antibacterial activity. Several works have shown that CPT alone, or in combination with other drugs, can effectively act as an antibiotic against various infectious bacteria. Some authors have related the mechanism underlying this action to CPT-membrane interaction. This work shows that CPT readily partitions into phosphatidylcholine membranes, but there is a limiting capacity of accommodation of ca. 1 mol CPT to 3 mol phospholipid. The addition of CPT to unilamellar liposomes composed of 1-palmitoyl-2-oleoylphosphatidylcholine (POPC) causes membrane permeabilization, as shown by fluorescent probe leakage. This process has been kinetically studied, as well as its modulation by incorporation of phosphatidylethanolamine or phosphatidylglycerol, as a model for pathogenic cell membranes. The thermotropic behavior of 1,2-dimyristoylphosphatidylcholine (DMPC) model membranes is weakly affected by CPT, but the terpenoid causes significant dehydration of the polar region of the bilayer and weak disordering of the acyl chain palisade, as observed in Fourier-transform infrared spectroscopy (FTIR) results. Small-angle X-ray scattering (SAXS) shows that CPT increases DMPC bilayer thickness, which could be due to localization near the phospholipid/water interface. Molecular dynamics (MD) simulations show that the lateral diffusion coefficient of the phospholipid increases with the presence of CPT. CPT extends from the polar head region to the center of the bilayer, being centered between the carbonyl groups and the unsaturated region of the POPC, where there is greater overlap. Interestingly, the free energy profiles of a water molecule crossing the lipid membrane show that the POPC membrane becomes more permeable in the presence of CPT. In summary, our results show that CPT perturbs the physicochemical properties of the phospholipid membrane and compromises its barrier function, which could be of relevance to explain part of its antimicrobial or anticancer activities.
Keywords: cryptotanshinone; membrane permeabilization; molecular dynamics; phospholipid membranes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures







Similar articles
-
Interaction of an acidic sophorolipid biosurfactant with phosphatidylcholine model membranes.Colloids Surf B Biointerfaces. 2021 Nov;207:112029. doi: 10.1016/j.colsurfb.2021.112029. Epub 2021 Aug 8. Colloids Surf B Biointerfaces. 2021. PMID: 34399158
-
Effect of acetone accumulation on structure and dynamics of lipid membranes studied by molecular dynamics simulations.Comput Biol Chem. 2013 Oct;46:23-31. doi: 10.1016/j.compbiolchem.2013.04.005. Epub 2013 May 7. Comput Biol Chem. 2013. PMID: 23764528
-
The pore-forming activity of sticholysin I is enhanced by the presence of a phospholipid hydroperoxide in membrane.Toxicon. 2021 Dec;204:44-55. doi: 10.1016/j.toxicon.2021.10.012. Epub 2021 Nov 1. Toxicon. 2021. PMID: 34736955
-
On the Mechanism of Membrane Permeabilization by Tamoxifen and 4-Hydroxytamoxifen.Membranes (Basel). 2023 Feb 28;13(3):292. doi: 10.3390/membranes13030292. Membranes (Basel). 2023. PMID: 36984678 Free PMC article.
-
The interaction of the antimicrobial peptide gramicidin S with lipid bilayer model and biological membranes.Biochim Biophys Acta. 1999 Dec 15;1462(1-2):201-21. doi: 10.1016/s0005-2736(99)00207-2. Biochim Biophys Acta. 1999. PMID: 10590309 Review.
Cited by
-
Pharmacological Mechanisms of Cryptotanshinone: Recent Advances in Cardiovascular, Cancer, and Neurological Disease Applications.Drug Des Devel Ther. 2024 Dec 15;18:6031-6060. doi: 10.2147/DDDT.S494555. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39703195 Free PMC article. Review.
References
-
- Gao H., Huang L., Ding F., Yang K., Feng Y., Tang H., Xu Q.M., Feng J., Yang S. Simultaneous purification of dihydrotanshinone, tanshinone I, cryptotanshinone, and tanshinone IIA from Salvia miltiorrhiza and their anti-inflammatory activities investigation. Sci. Rep. 2018;8:8460. doi: 10.1038/s41598-018-26828-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

