Stem-Cell-Regenerative and Protective Effects of Squid (Symplectoteuthis oualaniensis) Skin Collagen Peptides against H2O2-Induced Fibroblast Injury
- PMID: 38921566
- PMCID: PMC11204806
- DOI: 10.3390/md22060255
Stem-Cell-Regenerative and Protective Effects of Squid (Symplectoteuthis oualaniensis) Skin Collagen Peptides against H2O2-Induced Fibroblast Injury
Abstract
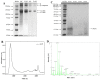
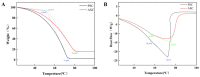
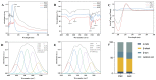
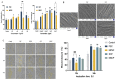
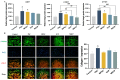
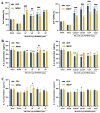
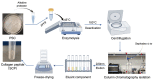
Recently, there has been a growing interest in collagen peptides derived from marine sources for their notable ability to protect skin cells against apoptosis induced by oxidants. Therefore, the current study aimed to investigate the fundamental properties of collagen peptides, including their physicochemical, thermal, structural, stem-cell-regenerative, and skin-cell-protective effects, in comparison to commercial collagen peptides. The acid-soluble (ASC) and pepsin-soluble (PSC) collagens exhibited three distinct bands on SDS-PAGE, namely α (α1 and α2), β, and γ chains, confirming a type I pattern. The thermal profiles obtained from TG and DSC analyses confirmed the denaturation of PSC and ASC at temperatures ranging from 51.94 to 56.4 °C and from 52.07 to 56.53 °C, respectively. The purified collagen peptides were analyzed using SDS-PAGE and MALDI-TOF mass spectrometry, revealing a mass range of 900-15,000 Da. Furthermore, the de novo peptide sequence analysis confirmed the presence of the Gly-X-Y repeating sequence in collagen peptides. Collagen peptide treatments significantly enhanced HFF-1 cell proliferation and migration compared to the control group. ELISA results confirmed the potential interactions between collagen peptides and HFF-1 cells through α2β1, α10β1, and α11β1 integrin receptors. Notably, collagen peptide treatment effectively restored the proliferation of HFF-1 cells damaged by H2O2. Consequently, the advantageous characteristics of squid skin collagen peptides highlight their promising role in regenerative medicine.
Keywords: H2O2 oxidative damage; regenerative medicine; skin cells; squid collagen peptides.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Polyethylene Glycolylation of the Purified Basic Protein (Protamine) of Squid (Symplectoteuthis oualaniensis): Structural Changes and Evaluation of Proliferative Effects on Fibroblast.Int J Mol Sci. 2025 Feb 21;26(5):1869. doi: 10.3390/ijms26051869. Int J Mol Sci. 2025. PMID: 40076495 Free PMC article.
-
Molecular and physical characteristics of squid (Todarodes pacificus) skin collagens and biological properties of their enzymatic hydrolysates.J Food Sci. 2008 May;73(4):C249-55. doi: 10.1111/j.1750-3841.2008.00722.x. J Food Sci. 2008. PMID: 18460118
-
Isolation and structural characterisation of acid- and pepsin-soluble collagen from the skin of squid Sepioteuthis lessoniana (Lesson, 1830).Nat Prod Res. 2014;28(11):838-42. doi: 10.1080/14786419.2014.880914. Epub 2014 Jan 31. Nat Prod Res. 2014. PMID: 24483725
-
Comparison of Physicochemical and Structural Properties of Acid-Soluble and Pepsin-Soluble Collagens from Blacktip Reef Shark Skin.Mar Drugs. 2022 Jun 2;20(6):376. doi: 10.3390/md20060376. Mar Drugs. 2022. PMID: 35736179 Free PMC article.
-
Preparation of Antioxidant Peptide by Microwave- Assisted Hydrolysis of Collagen and Its Protective Effect Against H2O2-Induced Damage of RAW264.7 Cells.Mar Drugs. 2019 Nov 14;17(11):642. doi: 10.3390/md17110642. Mar Drugs. 2019. PMID: 31739542 Free PMC article.
Cited by
-
Polyethylene Glycolylation of the Purified Basic Protein (Protamine) of Squid (Symplectoteuthis oualaniensis): Structural Changes and Evaluation of Proliferative Effects on Fibroblast.Int J Mol Sci. 2025 Feb 21;26(5):1869. doi: 10.3390/ijms26051869. Int J Mol Sci. 2025. PMID: 40076495 Free PMC article.
-
Immunomodulatory Effects of Symplectoteuthis oualaniensis Protamine and Its PEG Derivative on Macrophages: Involvement of PI3K/Akt Signaling, Redox Regulation, and Cell Cycle Modulation.Antioxidants (Basel). 2025 Apr 4;14(4):437. doi: 10.3390/antiox14040437. Antioxidants (Basel). 2025. PMID: 40298789 Free PMC article.
-
Monitoring fish freshness with pH-sensitive hydrogel films containing quercetin or eucalyptol.Food Chem X. 2024 Aug 22;23:101738. doi: 10.1016/j.fochx.2024.101738. eCollection 2024 Oct 30. Food Chem X. 2024. PMID: 39257495 Free PMC article.
-
Comparative analysis of antioxidant activity and accelerated collagen expression of human skin fibroblasts with gelatin hydrolysates from different sources.Food Chem X. 2025 Jul 29;29:102850. doi: 10.1016/j.fochx.2025.102850. eCollection 2025 Jul. Food Chem X. 2025. PMID: 40791881 Free PMC article.
References
-
- Ahmed M., Verma A.K., Patel R. Collagen Extraction and Recent Biological Activities of Collagen Peptides Derived from Sea-Food Waste: A Review. Sustain. Chem. Pharm. 2020;18:100315. doi: 10.1016/j.scp.2020.100315. - DOI
-
- Jeevithan E., Bao B., Zhang J., Hong S., Wu W. Purification, Characterization and Antioxidant Properties of Low Molecular Weight Collagenous Polypeptide (37 kDa) Prepared from Whale Shark Cartilage (Rhincodon typus) J. Food Sci. Technol. 2015;52:6312–6322. doi: 10.1007/s13197-015-1715-5. - DOI - PMC - PubMed
-
- Jeevithan E., Jingyi Z., Bao B., Shujun W., JeyaShakila R., Wu W.H. Biocompatibility Assessment of Type-II Collagen and Its Polypeptide for Tissue Engineering: Effect of Collagen’s Molecular Weight and Glycoprotein Content on Tumor Necrosis Factor (Fas/Apo-1) Receptor Activation in Human Acute T-Lymphocyte Leukemia Cell Line. RSC Adv. 2016;6:14236–14246. doi: 10.1039/C5RA24979A. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

