A PCR Test Using the Mini-PCR Platform and Simplified Product Detection Methods Is Highly Sensitive and Specific to Detect Fasciola hepatica DNA Mixed in Human Stool, Snail Tissue, and Water DNA Specimens
- PMID: 38921738
- PMCID: PMC11206539
- DOI: 10.3390/pathogens13060440
A PCR Test Using the Mini-PCR Platform and Simplified Product Detection Methods Is Highly Sensitive and Specific to Detect Fasciola hepatica DNA Mixed in Human Stool, Snail Tissue, and Water DNA Specimens
Abstract
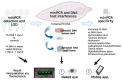
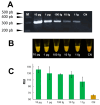
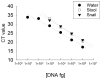
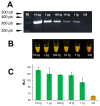
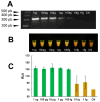
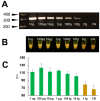
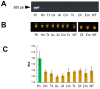
Fasciola hepatica has a complex lifecycle with multiple intermediate and definitive hosts and influenced by environmental factors. The disease causes significant morbidity in children and its prevalent worldwide. There is lack of data about distribution and burden of the disease in endemic regions, owing to poor efficacy of the different diagnostic methods used. A novel PCR-based test was developed by using a portable mini-PCR® platform to detect Fasciola sp. DNA and interpret the results via a fluorescence viewer and smartphone image analyzer application. Human stool, snail tissue, and water samples were used to extract DNA. Primers targeting the ITS-1 of the 18S rDNA gene of Fasciola sp. were used. The limit of detection of the mini-PCR test was 1 fg/μL for DNA samples diluted in water, 10 fg/μL for Fasciola/snail DNA scramble, and 100 fg/μL for Fasciola/stool DNA scramble. The product detection by agarose gel, direct visualization, and image analyses showed the same sensitivity. The Fh mini-PCR had a sensitivity and specificity equivalent to real-time PCR using the same specimens. Testing was also done on infected human stool and snail tissue successfully. These experiments demonstrated that Fh mini-PCR is as sensitive and specific as real time PCR but without the use of expensive equipment and laboratory facilities. Further testing of multiple specimens with natural infection will provide evidence for feasibility of deployment to resource constrained laboratories.
Keywords: Fasciola hepatica; ITS-1 gene; RT-PCR; mini-PCR; molecular diagnostics.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures
Similar articles
-
Development of a multiplex quantitative PCR assay for detection and quantification of DNA from Fasciola hepatica and the intermediate snail host, Austropeplea tomentosa, in water samples.Vet Parasitol. 2018 Aug 15;259:17-24. doi: 10.1016/j.vetpar.2018.06.018. Epub 2018 Jun 24. Vet Parasitol. 2018. PMID: 30056979
-
Development of a nest-PCR for detection of Fasciola hepatica DNA in the intermediate snail host, Radix cucunorica, and the prevalence in northwestern China.Infect Genet Evol. 2019 Nov;75:103984. doi: 10.1016/j.meegid.2019.103984. Epub 2019 Jul 29. Infect Genet Evol. 2019. PMID: 31369864
-
Recombinase Polymerase Amplification Compared to Real-Time Polymerase Chain Reaction Test for the Detection of Fasciola hepatica in Human Stool.Am J Trop Med Hyg. 2017 Feb 8;96(2):341-346. doi: 10.4269/ajtmh.16-0601. Epub 2016 Nov 7. Am J Trop Med Hyg. 2017. PMID: 27821691 Free PMC article.
-
Development and validation of a mtDNA multiplex PCR for identification and discrimination of Calicophoron daubneyi and Fasciola hepatica in the Galba truncatula snail.Vet Parasitol. 2013 Jul 1;195(1-2):57-64. doi: 10.1016/j.vetpar.2012.12.048. Epub 2013 Jan 3. Vet Parasitol. 2013. PMID: 23333073
-
Chapter 2. Fasciola, lymnaeids and human fascioliasis, with a global overview on disease transmission, epidemiology, evolutionary genetics, molecular epidemiology and control.Adv Parasitol. 2009;69:41-146. doi: 10.1016/S0065-308X(09)69002-3. Adv Parasitol. 2009. PMID: 19622408 Review.
References
-
- Fascioliasis Pan American Health Organization (PAHO) [(accessed on 26 April 2024)]. Available online: www.paho.org/en/topics/fascioliasis.
-
- Pan American Health Organization (PAHO) Meeting Report of the PAHO Strategic and Technical Advisory Group on Disease Elimination. [(accessed on 26 April 2024)]. Meeting Report and Recommendations. 29–30 November 2022. Washington, D.C, 2023. Available online: https://iris.paho.org/handle/10665.2/58002.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

