Adaptive Cellular Responses following SARS-CoV-2 Vaccination in Primary Antibody Deficiency Patients
- PMID: 38921811
- PMCID: PMC11206773
- DOI: 10.3390/pathogens13060514
Adaptive Cellular Responses following SARS-CoV-2 Vaccination in Primary Antibody Deficiency Patients
Abstract
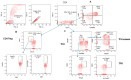
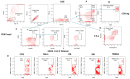
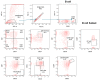
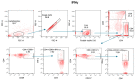
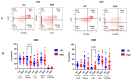
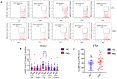
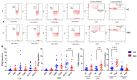
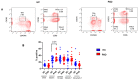
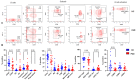
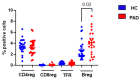
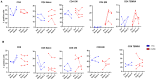
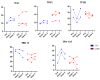
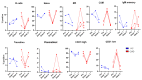
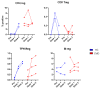
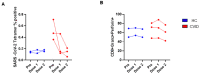
Since the start of the COVID-19 pandemic, in a short span of 3 years, vaccination against SARS-CoV-2 has resulted in the end of the pandemic. Patients with inborn errors of immunity (IEI) are at an increased risk for SARS-CoV-2 infection; however, serious illnesses and mortality, especially in primary antibody deficiencies (PADs), have been lower than expected and lower than other high-risk groups. This suggests that PAD patients may mount a reasonable effective response to the SARS-CoV-2 vaccine. Several studies have been published regarding antibody responses, with contradictory reports. The current study is, perhaps, the most comprehensive study of phenotypically defined various lymphocyte populations in PAD patients following the SARS-CoV-2 vaccine. In this study, we examined, following two vaccinations and, in a few cases, prior to and following the 1st and 2nd vaccinations, subsets of CD4 and CD8 T cells (Naïve, TCM, TEM, TEMRA), T follicular helper cells (TFH1, TFH2, TFH17, TFH1/17), B cells (naïve, transitional, marginal zone, germinal center, IgM memory, switched memory, plasmablasts, CD21low), regulatory lymphocytes (CD4Treg, CD8Treg, TFR, Breg), and SARS-CoV-2-specific activation of CD4 T cells and CD8 T cells (CD69, CD137), SARS-CoV-2 tetramer-positive CD8 T cells, and CD8 CTL. Our data show significant alterations in various B cell subsets including Breg, whereas only a few subsets of various T cells revealed alterations. These data suggest that large proportions of PAD patients may mount significant responses to the vaccine.
Keywords: Breg; CD4 Treg; CD8 Treg; COVID-19 vaccine; CVID; Cytotoxic T cells; IFNγ; TFR.
Conflict of interest statement
All authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results in Pathogens.
Figures
References
-
- Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. - DOI - PMC - PubMed
-
- McMenamin M.E., Nealon J., Lin Y., Wong J.Y., Cheung J.K., Lau E.H.Y., Wu P., Leung G.M., Cowling B.J. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: A population-based observational study. Lancet Infect. Dis. 2022;22:1435–1443. doi: 10.1016/S1473-3099(22)00345-0. - DOI - PMC - PubMed
-
- Tarke A., Sidney J., Kidd C.K., Dan J.M., Ramirez S.I., Yu E.D., Mateus J., da Silva Antunes R., Moore E., Rubiro P., et al. Comprehensive analysis of T cell immunodominance and immunoprevalence of SARS-CoV-2 epitopes in COVID-19 cases. Cell Rep. Med. 2021;2:100204. doi: 10.1016/j.xcrm.2021.100204. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

