Boosting PRRSV-Specific Cellular Immunity: The Immunological Profiling of an Fc-Fused Multi-CTL Epitope Vaccine in Mice
- PMID: 38922021
- PMCID: PMC11209284
- DOI: 10.3390/vetsci11060274
Boosting PRRSV-Specific Cellular Immunity: The Immunological Profiling of an Fc-Fused Multi-CTL Epitope Vaccine in Mice
Abstract
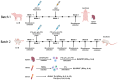
The continuously evolving PRRSV has been plaguing pig farms worldwide for over 30 years, with conventional vaccines suffering from insufficient protection and biosecurity risks. To address these challenges, we identified 10 PRRSV-specific CTL epitopes through enzyme-linked immunospot assay (ELISPOT) and constructed a multi-epitope peptide (PTE) by linking them in tandem. This PTE was then fused with a modified porcine Fc molecule to create the recombinant protein pFc-PTE. Our findings indicate that pFc-PTE effectively stimulates PRRSV-infected specific splenic lymphocytes to secrete high levels of interferon-gamma (IFN-γ) and is predicted to be non-toxic and non-allergenic. Compared to PTE alone, pFc-PTE not only induced a comparable cellular immune response in mice but also extended the duration of the immune response to at least 10 weeks post-immunization. Additionally, pFc-PTE predominantly induced a Th1 immune response, suggesting its potential advantage in enhancing cellular immunity. Consequently, pFc-PTE holds promise as a novel, safe, and potent candidate vaccine for PRRSV and may also provide new perspectives for vaccine design against other viral diseases.
Keywords: CTL epitope; Fc; cellular immunity; porcine reproductive and respiratory syndrome virus (PRRSV); vaccine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A candidate multi-epitope vaccine against porcine reproductive and respiratory syndrome virus and Mycoplasma hyopneumoniae induces robust humoral and cellular response in mice.Vaccine. 2022 Apr 6;40(16):2370-2378. doi: 10.1016/j.vaccine.2022.03.021. Epub 2022 Mar 17. Vaccine. 2022. PMID: 35307227
-
Replacing the decoy epitope of PCV2 capsid protein with epitopes of GP3 and/or GP5 of PRRSV enhances the immunogenicity of bivalent vaccines in mice.J Virol Methods. 2020 Oct;284:113928. doi: 10.1016/j.jviromet.2020.113928. Epub 2020 Jul 7. J Virol Methods. 2020. PMID: 32650038
-
Lymphocyte activation as cytokine gene expression and secretion is related to the porcine reproductive and respiratory syndrome virus (PRRSV) isolate after in vitro homologous and heterologous recall of peripheral blood mononuclear cells (PBMC) from pigs vaccinated and exposed to natural infection.Vet Immunol Immunopathol. 2013 Feb 15;151(3-4):193-206. doi: 10.1016/j.vetimm.2012.11.006. Epub 2012 Nov 19. Vet Immunol Immunopathol. 2013. PMID: 23228653
-
Identification of Novel T-Cell Epitopes on Infectious Bronchitis Virus N Protein and Development of a Multi-epitope Vaccine.J Virol. 2021 Aug 10;95(17):e0066721. doi: 10.1128/JVI.00667-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34105997 Free PMC article.
-
Innate and adaptive immunity against Porcine Reproductive and Respiratory Syndrome Virus.Vet Immunol Immunopathol. 2015 Sep 15;167(1-2):1-14. doi: 10.1016/j.vetimm.2015.07.003. Epub 2015 Jul 17. Vet Immunol Immunopathol. 2015. PMID: 26209116 Free PMC article. Review.
Cited by
-
Strategies and scheming: the war between PRRSV and host cells.Virol J. 2025 Jun 11;22(1):191. doi: 10.1186/s12985-025-02685-y. Virol J. 2025. PMID: 40500743 Free PMC article. Review.
-
Current Status of Porcine Reproductive and Respiratory Syndrome Vaccines.Vaccines (Basel). 2024 Dec 10;12(12):1387. doi: 10.3390/vaccines12121387. Vaccines (Basel). 2024. PMID: 39772049 Free PMC article. Review.
-
A Universal Multi-Epitope Vaccine Design Against Porcine Reproductive and Respiratory Syndrome Virus via Bioinformatics and Immunoinformatics Approaches.Vet Sci. 2024 Dec 16;11(12):659. doi: 10.3390/vetsci11120659. Vet Sci. 2024. PMID: 39728999 Free PMC article.
References
-
- Wang J., Zhang M., Cui X., Gao X., Sun W., Ge X., Zhang Y., Guo X., Han J., Zhou L., et al. Attenuated Porcine Reproductive and Respiratory Syndrome Virus Regains Its Fatal Virulence by Serial Passaging in Pigs or Porcine Alveolar Macrophages To Increase Its Adaptation to Target Cells. Microbiol. Spectr. 2022;10:e0308422. doi: 10.1128/spectrum.03084-22. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

