Tumor-Agnostic Genomic and Clinical Analysis of BRAF Fusions Identifies Actionable Targets
- PMID: 38922339
- PMCID: PMC11371517
- DOI: 10.1158/1078-0432.CCR-23-3981
Tumor-Agnostic Genomic and Clinical Analysis of BRAF Fusions Identifies Actionable Targets
Abstract
Purpose: Even though BRAF fusions are increasingly detected in standard multigene next-generation sequencing panels, few reports have explored their structure and impact on clinical course.
Experimental design: We collected data from patients with BRAF fusion-positive cancers identified through a genotyping protocol of 97,024 samples. Fusions were characterized and reviewed for oncogenic potential (in-frame status, non-BRAF partner gene, and intact BRAF kinase domain).
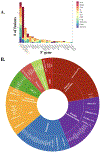
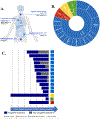
Results: We found 241 BRAF fusion-positive tumors from 212 patients with 82 unique 5' fusion partners spanning 52 histologies. Thirty-nine fusion partners were not previously reported, and 61 were identified once. BRAF fusion incidence was enriched in pilocytic astrocytomas, gangliogliomas, low-grade neuroepithelial tumors, and acinar cell carcinoma of the pancreas. Twenty-four patients spanning multiple histologies were treated with MAPK-directed therapies, of which 20 were evaluable for RECIST. Best response was partial response (N = 2), stable disease (N = 11), and progressive disease (N = 7). The median time on therapy was 1 month with MEK plus BRAF inhibitors [(N = 11), range 0-18 months] and 8 months for MEK inhibitors [(N = 14), range 1-26 months]. Nine patients remained on treatment for longer than 6 months [pilocytic astrocytomas (N = 6), Erdheim-Chester disease (N = 1), extraventricular neurocytoma (N = 1), and melanoma (N = 1)]. Fifteen patients had acquired BRAF fusions.
Conclusions: BRAF fusions are found across histologies and represent an emerging actionable target. BRAF fusions have a diverse set of fusion partners. Durable responses to MAPK therapies were seen, particularly in pilocytic astrocytomas. Acquired BRAF fusions were identified after targeted therapy, underscoring the importance of postprogression biopsies to optimize treatment at relapse in these patients.
©2024 American Association for Cancer Research.
Figures


References
-
- Yaeger R, Corcoran RB. Targeting Alterations in the RAF–MEK Pathway. Cancer Discovery 2019;9(3):329–41 doi 10.1158/2159-8290.Cd-18-1321. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

