Trimethoprim-sulfamethoxazole dosing and outcomes of pulmonary nocardiosis
- PMID: 38922564
- PMCID: PMC11825568
- DOI: 10.1007/s15010-024-02323-9
Trimethoprim-sulfamethoxazole dosing and outcomes of pulmonary nocardiosis
Abstract
Background: Nocardia often causes pulmonary infection among those with chronic pulmonary disease or immunocompromising conditions. Trimethoprim-sulfamethoxazole (TMP-SMX) is recommended as first-line treatment, though little data exists regarding outcomes of different dosing regimens.
Methods: We performed a multicenter retrospective cohort study of adult patients with non-disseminated pulmonary nocardiosis initially treated with TMP-SMX monotherapy. Patients' initial TMP-SMX dosing was categorized as high- (> 10 mg/kg/day), intermediate- (5-10 mg/kg/day) or low-dose (< 5 mg/kg/day). Outcomes included one-year mortality, post-treatment recurrence, and dose adjustment or early discontinuation of TMP-SMX. SMX serum concentrations and their effect on management were also assessed. Inverse probability of treatment weighting was applied to Cox regression analyses.
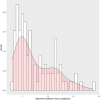
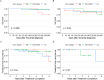
Results: Ninety-one patients were included with 24 (26.4%), 37 (40.7%), and 30 (33.0%) treated with high-, intermediate-, and low-dose TMP-SMX, respectively. Patients who initially received low-dose (HR 0.07, 95% CI 0.01-0.68) and intermediate-dose TMP-SMX (HR 0.27, 95% CI 0.07-1.04) had lower risk of one-year mortality than the high-dose group. Risk of recurrence was similar between groups. Nineteen patients had peak SMX serum concentrations measured which resulted in 7 (36.8%) dose changes and was not associated with one-year mortality or recurrence. However, 66.7% of the high-dose group required TMP-SMX dose adjustment/discontinuation compared to 24.3% of the intermediate-dose and 26.7% of the low-dose groups (p = 0.001).
Conclusions: Low- and intermediate-dose TMP-SMX for non-disseminated pulmonary nocardiosis were not associated with poor outcomes compared to high-dose therapy, which had a higher rate of dose adjustment/early discontinuation. Historically used high-dose TMP-SMX may not be necessary for management of isolated pulmonary nocardiosis.
Keywords: Nocardia; Bactrim; Bronchiectasis; Dose; Sulfonamide.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: All authors have no conflicts of interest to report.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

