Concordance With Screening and Treatment Guidelines for Chronic Kidney Disease in Type 2 Diabetes
- PMID: 38922613
- PMCID: PMC11208975
- DOI: 10.1001/jamanetworkopen.2024.18808
Concordance With Screening and Treatment Guidelines for Chronic Kidney Disease in Type 2 Diabetes
Abstract
Importance: Chronic kidney disease (CKD) is an often-asymptomatic complication of type 2 diabetes (T2D) that requires annual screening to diagnose. Patient-level factors linked to inadequate screening and treatment can inform implementation strategies to facilitate guideline-recommended CKD care.
Objective: To identify risk factors for nonconcordance with guideline-recommended CKD screening and treatment in patients with T2D.
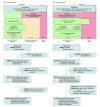
Design, setting, and participants: This retrospective cohort study was performed at 20 health care systems contributing data to the US National Patient-Centered Clinical Research Network. To evaluate concordance with CKD screening guidelines, adults with an outpatient clinician visit linked to T2D diagnosis between January 1, 2015, and December 31, 2020, and without known CKD were included. A separate analysis reviewed prescription of angiotensin-converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) and sodium-glucose cotransporter 2 (SGLT2) inhibitors in adults with CKD (estimated glomerular filtration rate [eGFR] of 30-90 mL/min/1.73 m2 and urinary albumin-to-creatinine ratio [UACR] of 200-5000 mg/g) and an outpatient clinician visit for T2D between October 1, 2019, and December 31, 2020. Data were analyzed from July 8, 2022, through June 22, 2023.
Exposures: Demographics, lifestyle factors, comorbidities, medications, and laboratory results.
Main outcomes and measures: Screening required measurement of creatinine levels and UACR within 15 months of the index visit. Treatment reflected prescription of ACEIs or ARBs and SGLT2 inhibitors within 12 months before or 6 months following the index visit.
Results: Concordance with CKD screening guidelines was assessed in 316 234 adults (median age, 59 [IQR, 50-67] years), of whom 51.5% were women; 21.7%, Black; 10.3%, Hispanic; and 67.6%, White. Only 24.9% received creatinine and UACR screening, 56.5% received 1 screening measurement, and 18.6% received neither. Hispanic ethnicity was associated with lack of screening (relative risk [RR], 1.16 [95% CI, 1.14-1.18]). In contrast, heart failure, peripheral arterial disease, and hypertension were associated with a lower risk of nonconcordance. In 4215 patients with CKD and albuminuria, 3288 (78.0%) received an ACEI or ARB; 194 (4.6%), an SGLT2 inhibitor; and 885 (21.0%), neither therapy. Peripheral arterial disease and lower eGFR were associated with lack of CKD treatment, while diuretic or statin prescription and hypertension were associated with treatment.
Conclusions and relevance: In this cohort study of patients with T2D, fewer than one-quarter received recommended CKD screening. In patients with CKD and albuminuria, 21.0% did not receive an SGLT2 inhibitor or an ACEI or an ARB, despite compelling indications. Patient-level factors may inform implementation strategies to improve CKD screening and treatment in people with T2D.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention . National diabetes statistics report. Reviewed November 29, 2023. Accessed April 6, 2023. https://www.cdc.gov/diabetes/php/data-research/?CDC_AAref_Val=https://ww...
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group . KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1-S115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

