Examining Associations Between Smartphone Use and Clinical Severity in Frontotemporal Dementia: Proof-of-Concept Study
- PMID: 38922667
- PMCID: PMC11237775
- DOI: 10.2196/52831
Examining Associations Between Smartphone Use and Clinical Severity in Frontotemporal Dementia: Proof-of-Concept Study
Abstract
Background: Frontotemporal lobar degeneration (FTLD) is a leading cause of dementia in individuals aged <65 years. Several challenges to conducting in-person evaluations in FTLD illustrate an urgent need to develop remote, accessible, and low-burden assessment techniques. Studies of unobtrusive monitoring of at-home computer use in older adults with mild cognitive impairment show that declining function is reflected in reduced computer use; however, associations with smartphone use are unknown.
Objective: This study aims to characterize daily trajectories in smartphone battery use, a proxy for smartphone use, and examine relationships with clinical indicators of severity in FTLD.
Methods: Participants were 231 adults (mean age 52.5, SD 14.9 years; n=94, 40.7% men; n=223, 96.5% non-Hispanic White) enrolled in the Advancing Research and Treatment of Frontotemporal Lobar Degeneration (ARTFL study) and Longitudinal Evaluation of Familial Frontotemporal Dementia Subjects (LEFFTDS study) Longitudinal Frontotemporal Lobar Degeneration (ALLFTD) Mobile App study, including 49 (21.2%) with mild neurobehavioral changes and no functional impairment (ie, prodromal FTLD), 43 (18.6%) with neurobehavioral changes and functional impairment (ie, symptomatic FTLD), and 139 (60.2%) clinically normal adults, of whom 55 (39.6%) harbored heterozygous pathogenic or likely pathogenic variants in an autosomal dominant FTLD gene. Participants completed the Clinical Dementia Rating plus National Alzheimer's Coordinating Center Frontotemporal Lobar Degeneration Behavior and Language Domains (CDR+NACC FTLD) scale, a neuropsychological battery; the Neuropsychiatric Inventory; and brain magnetic resonance imaging. The ALLFTD Mobile App was installed on participants' smartphones for remote, passive, and continuous monitoring of smartphone use. Battery percentage was collected every 15 minutes over an average of 28 (SD 4.2; range 14-30) days. To determine whether temporal patterns of battery percentage varied as a function of disease severity, linear mixed effects models examined linear, quadratic, and cubic effects of the time of day and their interactions with each measure of disease severity on battery percentage. Models covaried for age, sex, smartphone type, and estimated smartphone age.
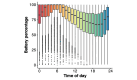
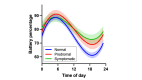
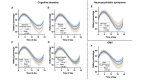
Results: The CDR+NACC FTLD global score interacted with time on battery percentage such that participants with prodromal or symptomatic FTLD demonstrated less change in battery percentage throughout the day (a proxy for less smartphone use) than clinically normal participants (P<.001 in both cases). Additional models showed that worse performance in all cognitive domains assessed (ie, executive functioning, memory, language, and visuospatial skills), more neuropsychiatric symptoms, and smaller brain volumes also associated with less battery use throughout the day (P<.001 in all cases).
Conclusions: These findings support a proof of concept that passively collected data about smartphone use behaviors associate with clinical impairment in FTLD. This work underscores the need for future studies to develop and validate passive digital markers sensitive to longitudinal clinical decline across neurodegenerative diseases, with potential to enhance real-world monitoring of neurobehavioral change.
Keywords: clinical trials; cognition; cognitive impairment; digital; mobile phone; monitoring; neurodegenerative; neuropsychology; remote; screening; technology.
©Emily W Paolillo, Kaitlin B Casaletto, Annie L Clark, Jack C Taylor, Hilary W Heuer, Amy B Wise, Sreya Dhanam, Mark Sanderson-Cimino, Rowan Saloner, Joel H Kramer, John Kornak, Walter Kremers, Leah Forsberg, Brian Appleby, Ece Bayram, Andrea Bozoki, Danielle Brushaber, R Ryan Darby, Gregory S Day, Bradford C Dickerson, Kimiko Domoto-Reilly, Fanny Elahi, Julie A Fields, Nupur Ghoshal, Neill Graff-Radford, Matthew G H Hall, Lawrence S Honig, Edward D Huey, Maria I Lapid, Irene Litvan, Ian R Mackenzie, Joseph C Masdeu, Mario F Mendez, Carly Mester, Toji Miyagawa, Georges Naasan, Belen Pascual, Peter Pressman, Eliana Marisa Ramos, Katherine P Rankin, Jessica Rexach, Julio C Rojas, Lawren VandeVrede, Bonnie Wong, Zbigniew K Wszolek, Bradley F Boeve, Howard J Rosen, Adam L Boxer, Adam M Staffaroni, ALLFTD Consortium. Originally published in JMIR Aging (https://aging.jmir.org), 26.06.2024.
Conflict of interest statement
Conflicts of Interest: BA has received research funding from the National Institutes of Health (NIH), the Centers for Disease Control and Prevention, the CJD Foundation, Alector, and Ionis; consulting fees from Acadia, Ionis, Sangamo, Gate, and Merck; and royalties from Wolters Kluwer. GSD reports no conflicts of interest that are directly relevant to this work. His research is supported by the NIH (K23AG064029, U01AG057195, U01NS120901, and U19AG032438), the Alzheimer’s Association, and the Chan Zuckerberg Initiative. He serves as a consultant for Parabon Nanolabs Inc; as a topic editor (dementia) for DynaMed (EBSCO); and as the clinical director of the Anti-NMDA Receptor Encephalitis Foundation Inc, Canada (uncompensated). He is the co–project principal investigator for a clinical trial in anti–N-methyl-D-aspartate (NMDA) receptor encephalitis, which receives support from Horizon Pharmaceuticals. He has developed educational materials for PeerView, and Continuing Education Inc. He owns stock in ANI Pharmaceuticals. His institution has received support from Eli Lilly for development and participation in an educational event promoting early diagnosis of symptomatic Alzheimer disease and in-kind contributions of radiotracer precursors for tau–positron emission tomography (PET) neuroimaging in studies of memory and aging (via Avid Radiopharmaceuticals, a wholly owned subsidiary of Eli Lilly). NG has participated, or is currently participating, in clinical trials of antidementia drugs sponsored by Bristol Myers Squibb, Eli Lilly and Avid Radiopharmaceuticals, Janssen Immunotherapy, Novartis, Pfizer, and Wyeth, as well as the Study of Nasal Insulin to Fight Forgetfulness (SNIFF) and the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) trial. She receives research support from the Tau Consortium and the Association for Frontotemporal Dementia and is funded by the NIH. IL’s research is supported by the NIH (2R01AG038791-06A, U01NS100610, R25NS098999, U19 AG063911-1, and 1R21NS114764-01A1), the Michael J Fox Foundation, the Parkinson’s Foundation, the Lewy Body Dementia Association, CurePSP, Roche, Abbvie, Biogen, Centogene, EIP Pharma, Biohaven Pharmaceuticals, Novartis, United BioPharma, and UCB. She is a member of the scientific advisory board for Amydis but does not receive funds and from the Rossy Progressive Supranuclear Palsy Program at the University of Toronto. She receives her salary from the University of California San Diego and as chief editor of Frontiers in Neurology. JCR is the site principal investigator for clinical trials sponsored by Eli Lilly and Eisai. He receives consulting fees from Roon. LVV is the site principal investigator for clinical trials sponsored by Biogen and has consulted for Retrotope. All other authors declare no other conflicts of interest.
Figures
References
-
- Knopman DS, Roberts RO. Estimating the number of persons with frontotemporal lobar degeneration in the US population. J Mol Neurosci. 2011 Nov 17;45(3):330–5. doi: 10.1007/s12031-011-9538-y. https://europepmc.org/abstract/MED/21584654 - DOI - PMC - PubMed
-
- Hendriks S, Peetoom K, Bakker C, van der Flier WM, Papma JM, Koopmans R, Verhey FR, de Vugt M, Köhler S, Young-Onset Dementia Epidemiology Study Group. Withall A, Parlevliet JL, Uysal-Bozkir Ö, Gibson RC, Neita SM, Nielsen TR, Salem LC, Nyberg J, Lopes MA, Dominguez JC, De Guzman MF, Egeberg A, Radford K, Broe T, Subramaniam M, Abdin E, Bruni AC, Di Lorenzo R, Smith K, Flicker L, Mol MO, Basta M, Yu D, Masika G, Petersen MS, Ruano L. Global prevalence of young-onset dementia: a systematic review and meta-analysis. JAMA Neurol. 2021 Sep 01;78(9):1080–90. doi: 10.1001/jamaneurol.2021.2161. https://europepmc.org/abstract/MED/34279544 2781919 - DOI - PMC - PubMed
-
- Perry D, Brown JA, Possin KL, Datta S, Trujillo A, Radke A, Karydas A, Kornak J, Sias AC, Rabinovici GD, Gorno-Tempini ML, Boxer AL, De May M, Rankin KP, Sturm VE, Lee SE, Matthews BR, Kao AW, Vossel KA, Tartaglia MC, Miller ZA, Seo SW, Sidhu M, Gaus SE, Nana AL, Vargas JN, Hwang JH, Ossenkoppele R, Brown AB, Huang EJ, Coppola G, Rosen HJ, Geschwind D, Trojanowski JQ, Grinberg LT, Kramer JH, Miller BL, Seeley WW. Clinicopathological correlations in behavioural variant frontotemporal dementia. Brain. 2017 Dec 01;140(12):3329–45. doi: 10.1093/brain/awx254. https://europepmc.org/abstract/MED/29053860 4371604 - DOI - PMC - PubMed
-
- Seo SW, Thibodeau MP, Perry DC, Hua A, Sidhu M, Sible I, Vargas JN, Gaus SE, Rabinovici GD, Rankin KD, Boxer AL, Kramer JH, Rosen HJ, Gorno-Tempini ML, Grinberg LT, Huang EJ, DeArmond SJ, Trojanowski JQ, Miller BL, Seeley WW. Early vs late age at onset frontotemporal dementia and frontotemporal lobar degeneration. Neurology. 2018 Mar 20;90(12):e1047–56. doi: 10.1212/wnl.0000000000005163. - DOI - PMC - PubMed
MeSH terms
Grants and funding
- K23 AG061253/AG/NIA NIH HHS/United States
- RF1 AG077557/AG/NIA NIH HHS/United States
- R01 AG058233/AG/NIA NIH HHS/United States
- K24 AG045333/AG/NIA NIH HHS/United States
- R01 AG038791/AG/NIA NIH HHS/United States
- RF1 AG032289/AG/NIA NIH HHS/United States
- P30 AG062422/AG/NIA NIH HHS/United States
- R01 AG032306/AG/NIA NIH HHS/United States
- K23 AG059888/AG/NIA NIH HHS/United States
- R01 AG032289/AG/NIA NIH HHS/United States
- P30 AG062677/AG/NIA NIH HHS/United States
- U01 AG045390/AG/NIA NIH HHS/United States
- U54 NS092089/NS/NINDS NIH HHS/United States
- P30 AG066514/AG/NIA NIH HHS/United States
- R25 NS098999/NS/NINDS NIH HHS/United States
- U01 NS100610/NS/NINDS NIH HHS/United States
- U24 AG021886/AG/NIA NIH HHS/United States
- U01 AG057195/AG/NIA NIH HHS/United States
- IK2 CX002180/CX/CSRD VA/United States
- U01 NS120901/NS/NINDS NIH HHS/United States
- R21 NS114764/NS/NINDS NIH HHS/United States
- U19 AG063911/AG/NIA NIH HHS/United States
- U01 AG016976/AG/NIA NIH HHS/United States
- P01 AG019724/AG/NIA NIH HHS/United States
- K08 NS105916/NS/NINDS NIH HHS/United States
- RF1 NS050915/NS/NINDS NIH HHS/United States
- K23 AG064029/AG/NIA NIH HHS/United States
- R56 NS050915/NS/NINDS NIH HHS/United States
- R01 NS050915/NS/NINDS NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- K23 AG073514/AG/NIA NIH HHS/United States
- U19 AG032438/AG/NIA NIH HHS/United States

