Metabolic dialogues: regulators of chimeric antigen receptor T cell function in the tumor microenvironment
- PMID: 38922759
- PMCID: PMC11223614
- DOI: 10.1002/1878-0261.13691
Metabolic dialogues: regulators of chimeric antigen receptor T cell function in the tumor microenvironment
Abstract
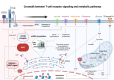
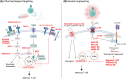
Tumor-infiltrating lymphocytes (TILs) and chimeric antigen receptor (CAR) T cells have demonstrated remarkable success in the treatment of relapsed/refractory melanoma and hematological malignancies, respectively. These treatments have marked a pivotal shift in cancer management. However, as "living drugs," their effectiveness is dependent on their ability to proliferate and persist in patients. Recent studies indicate that the mechanisms regulating these crucial functions, as well as the T cell's differentiation state, are conditioned by metabolic shifts and the distinct utilization of metabolic pathways. These metabolic shifts, conditioned by nutrient availability as well as cell surface expression of metabolite transporters, are coupled to signaling pathways and the epigenetic landscape of the cell, modulating transcriptional, translational, and post-translational profiles. In this review, we discuss the processes underlying the metabolic remodeling of activated T cells, the impact of a tumor metabolic environment on T cell function, and potential metabolic-based strategies to enhance T cell immunotherapy.
Keywords: T cells; anti‐tumor immunotherapy; chimeric antigen receptor; immunometabolism; nutrient transporters; tumor microenvironment.
© 2024 The Author(s). Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Almeida L, Lochner M, Berod L, Sparwasser T. Metabolic pathways in T cell activation and lineage differentiation. Semin Immunol. 2016;28(5):514–524. - PubMed
-
- Yong CS, Abba Moussa D, Cretenet G, Kinet S, Dardalhon V, Taylor N. Metabolic orchestration of T lineage differentiation and function. FEBS Lett. 2017;591(19):3104–3118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

