Free-breathing, fat-corrected T1 mapping of the liver with stack-of-stars MRI, and joint estimation of T1, PDFF, , and
- PMID: 38923009
- PMCID: PMC12207996
- DOI: 10.1002/mrm.30182
Free-breathing, fat-corrected T1 mapping of the liver with stack-of-stars MRI, and joint estimation of T1, PDFF, , and
Abstract
Purpose: Quantitative T1 mapping has the potential to replace biopsy for noninvasive diagnosis and quantitative staging of chronic liver disease. Conventional T1 mapping methods are confounded by fat and inhomogeneities, resulting in unreliable T1 estimations. Furthermore, these methods trade off spatial resolution and volumetric coverage for shorter acquisitions with only a few images obtained within a breath-hold. This work proposes a novel, volumetric (3D), free-breathing T1 mapping method to account for multiple confounding factors in a single acquisition.
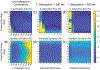
Theory and methods: Free-breathing, confounder-corrected T1 mapping was achieved through the combination of non-Cartesian imaging, magnetization preparation, chemical shift encoding, and a variable flip angle acquisition. A subspace-constrained, locally low-rank image reconstruction algorithm was employed for image reconstruction. The accuracy of the proposed method was evaluated through numerical simulations and phantom experiments with a T1/proton density fat fraction phantom at 3.0 T. Further, the feasibility of the proposed method was investigated through contrast-enhanced imaging in healthy volunteers, also at 3.0 T.
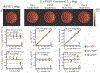
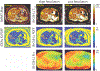
Results: The method showed excellent agreement with reference measurements in phantoms across a wide range of T1 values (200 to 1000 ms, slope = 0.998 (95% confidence interval (CI) [0.963 to 1.035]), intercept = 27.1 ms (95% CI [0.4 54.6]), r2 = 0.996), and a high level of repeatability. In vivo imaging studies demonstrated moderate agreement (slope = 1.099 (95% CI [1.067 to 1.132]), intercept = -96.3 ms (95% CI [-82.1 to -110.5]), r2 = 0.981) compared to saturation recovery-based T1 maps.
Conclusion: The proposed method produces whole-liver, confounder-corrected T1 maps through simultaneous estimation of T1, proton density fat fraction, and in a single, free-breathing acquisition and has excellent agreement with reference measurements in phantoms.
Keywords: T1 mapping; compressed‐sensing; free‐breathing imaging; inversion recovery; multi‐contrast; non‐Cartesian imaging.
© 2024 The Author(s). Magnetic Resonance in Medicine published by Wiley Periodicals LLC on behalf of International Society for Magnetic Resonance in Medicine.
Figures
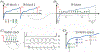


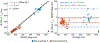
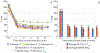
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

