The establishment of kidney cancer organoid line in drug testing
- PMID: 38923304
- PMCID: PMC11200131
- DOI: 10.1002/cam4.7432
The establishment of kidney cancer organoid line in drug testing
Abstract
Introduction: Kidney cancer is a common urological malignancy worldwide with an increasing incidence in recent years. Among all subtypes, renal cell carcinoma (RCC) represents the most predominant malignancy in kidney. Clinicians faced a major challenge to select the most effective and suitable treatment regime for patients from a wide range of modalities, despite improved understanding and diagnosis of RCC.
Objective: Recently, organoid culture gained more interest as the 3D model is shown to be highly patient specific which is hypothetically beneficial to the investigation of precision medicine. Nonetheless, the development and application of organotypic culture in RCC is still immature, therefore, the primary objective of this study was to establish an organoid model for RCC.
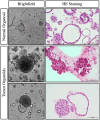
Materials and methods: Patients diagnosed with renal tumor and underwent surgical intervention were recruited. RCC specimen was collected and derived into organoids. Derived organoids were validated by histological examminations, sequencing and xenograft. Drug response of organoids were compared with resistance cell line and patients' clinical outcomes.
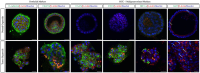
Results: Our results demonstrated that organoids could be successfully derived from renal tumor and they exhibited high concordance in terms of immunoexpressional patterns. Sequencing results also depicted concordant mutations of driver genes in both organoids and parental tumor tissues. Critical and novel growth factors were discovered during the establishment of organoid model. Besides, organoids derived from renal tumor exhibited tumorigenic properties in vivo. In addition, organoids recapitulated patient's in vivo drug resistance and served as a platform to predict responsiveness of other therapeutic agents.
Conclusion: Our RCC organoid model recaptiluated histological and genetic features observed in primary tumors. It also served as a potential platform in drug screening for RCC patients, though future studies are necessary before translating the outcomes into clinical practices.
Keywords: 3D culture; disease modeling; drug screening; organoid model; renal cell carcinoma.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






References
-
- Huang J, Leung DK, Chan EO, et al. A global trend analysis of kidney cancer incidence and mortality and their associations with smoking, alcohol consumption, and metabolic syndrome, Eur Urol. Focus. 2022;8:200‐209. - PubMed
-
- Cohen HT, McGovern FJ. Renal‐cell carcinoma. N Engl J Med. 2005;353:2477‐2490. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

