RECK/GPR124-driven WNT signaling in pancreatic and gastric cancer cells
- PMID: 38923741
- PMCID: PMC11462976
- DOI: 10.1111/cas.16258
RECK/GPR124-driven WNT signaling in pancreatic and gastric cancer cells
Abstract
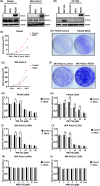
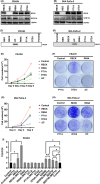
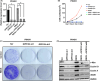
RECK has been described to modulate extracellular matrix components through negative regulation of MMP activities. Recently, RECK was demonstrated to bind to an orphan G protein-coupled receptor GPR124 to mediate WNT7 signaling in nontumor contexts. Here, we attempted to clarify the role of RECK in driving WNT signaling in cancer cells. RECK and GPR124 formed a complex in 293T cells, and when both were expressed, WNT signaling was significantly enhanced in a WNT7-dependent manner. This cooperation was abolished when RECK mutants unable to bind to GPR124 were transduced. RECK stimulated the growth of KRAS-mutated pancreatic ductal adenocarcinoma (PDAC) cells with increased sensitivity to WNT inhibitor in a GPR124-dependent manner. A gastric cancer cell line SH10TC endogenously expresses both RECK and GPR124 under regular culture conditions. In this cell line, inhibited cell growth and WNT signaling as well as increased apoptosis in the GPR124 depletion was dominantly found over those in the RECK deletion. These findings suggest that RECK promotes tumor cell growth by positively modulating WNT signaling through GPR124. This study proposes that the RECK/GPR124 complex might be a good therapeutic target in PDAC and gastric cancer.
Keywords: GPR124; RECK; WNT7; gastric cancer; pancreatic cancer.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no financial interest to disclose. Chiaki Takahashi is an editorial board member of
Figures


References
-
- Kitayama H, Sugimoto Y, Matsuzaki T, Ikawa Y, Noda M. A ras‐related gene with transformation suppressor activity. Cell. 1989;56:77‐84. - PubMed
-
- Chang HC, Cho CY, Hung WC. Silencing of the metastasis suppressor RECK by RAS oncogene is mediated by DNA Methyltransferase 3b–induced promoter methylation. Cancer Res. 2006;66:8413‐8420. - PubMed
-
- Sasahara RM, Takahashi C, Noda M. Involvement of the Sp1 site in ras‐mediated downregulation of the RECK metastasis suppressor gene. Biochem Biophys Res Commun. 1999;264:668‐675. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

