Attaching Metal-Containing Moieties to β-Lactam Antibiotics: The Case of Penicillin and Cephalosporin
- PMID: 38923955
- PMCID: PMC11234371
- DOI: 10.1021/acs.inorgchem.4c01548
Attaching Metal-Containing Moieties to β-Lactam Antibiotics: The Case of Penicillin and Cephalosporin
Abstract
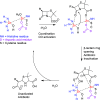
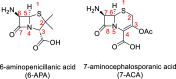
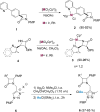
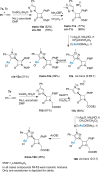
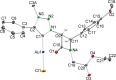
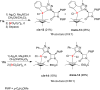
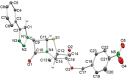
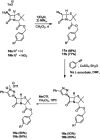
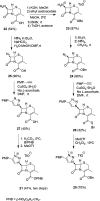
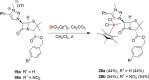
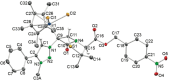
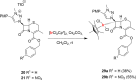
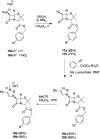
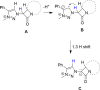
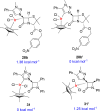
Procedures for the preparation of transition metal complexes having intact bicyclic cepham or penam systems as ligands have been developed. Starting from readily available 4-azido-2-azetidinones, a synthetic approach has been tuned using a copper-catalyzed azide-alkyne cycloaddition between 3-azido-2-azetinones and alkynes, followed by methylation and transmetalation to Au(I) and Ir(III) complexes from the mesoionic carbene Ag(I) complexes. This methodology was applied to 6-azido penam and 7-azido cepham derivatives to build 6-(1,2,3-triazolyl)penam and 7-(1,2,3-triazolyl)cepham proligands, which upon methylation and metalation with Au(I) and Ir(III) complexes yielded products derived from the coordination of the metal to the penam C6 and cepham C7 positions, preserving intact the bicyclic structure of the penicillin and cephalosporin scaffolds. The crystal structure of complex 28b, which has an Ir atom directly bonded to the intact penicillin bicycle, was determined by X-ray diffraction. This is the first structural report of a penicillin-transition-metal complex having the bicyclic system of these antibiotics intact. The selectivity of the coordination processes was interpreted using DFT calculations.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

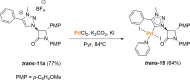
References
-
- O’Neill J.Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Government of the United Kingdom, 2016.
-
- Additional evidence from the World Health Organization (WHO) shows the extensive overuse of antibiotics during COVID-19 pandemic worldwide, which may have exacerbated ″silent″ spread of antimicrobial resistance (AMR). Published on WHO web page: https://www.who.int/news/item/26-04-2024-who-reports-widespread-overuse-... (accessed May 22, 2024).
- Langford B. J.; So M.; Simeonova M.; Leung V.; Lo J.; Kan T.; Raybardhan S.; Sapin M. E.; Mponponsuo K.; Farrell A.; Leung E.; Soucy J.-P. R.; Cassini A.; MacFadden D.; Daneman N.; Bertagnolio S. Antimicrobial Resistance in Patients with COVID-19: A Systematic Review and Meta-analysis. Lancet Microbe 2023, 4, E179–E191. 10.1016/S2666-5247(22)00355-X. - DOI - PMC - PubMed
-
-
The first β-lactamase was identified at the beginning of the 1940s decade, even before of the massive use of penicillin during and after WWII. See:
- Abraham E. Roots: Selective reminiscences of β-lactam antibiotics: Early research on penicillin and cephalosporins. BioEssays 1990, 12, 601–606. 10.1002/bies.950121208. - DOI - PubMed
- Anderson R. J.; Groundwater P. W.; Todd A.; Worsley A.. Antibacterial Agents: Chemistry, Mode of Action, Mechanisms of Resistance and Clinical Applications; Wiley: New York, 2012.
-
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

