A distal enhancer of GATA3 regulates Th2 differentiation and allergic inflammation
- PMID: 38923989
- PMCID: PMC11228505
- DOI: 10.1073/pnas.2320727121
A distal enhancer of GATA3 regulates Th2 differentiation and allergic inflammation
Abstract
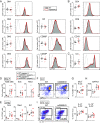
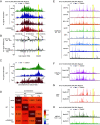
Asthma is a widespread airway disorder where GATA3-dependent Type-2 helper T (Th2) cells and group 2 innate lymphoid cells (ILC2s) play vital roles. Asthma-associated single nucleotide polymorphisms (SNPs) are enriched in a region located 926-970 kb downstream from GATA3 in the 10p14 (hG900). However, it is unknown how hG900 affects the pathogenesis of allergic airway inflammation. To investigate the roles of the asthma-associated GATA3 enhancer region in experimental allergic airway inflammation, we first examined the correlation between GATA3 expression and the activation of the hG900 region was analyzed by flow cytometry and ChIP-qPCR. We found that The activation of enhancers in the hG900 region was strongly correlated to the levels of GATA3 in human peripheral T cell subsets. We next generated mice lacking the mG900 region (mG900KO mice) were generated by the CRISPR-Cas9 system, and the development and function of helper T cells and ILCs in mG900KO mice were analyzed in steady-state conditions and allergic airway inflammation induced by papain or house dust mite (HDM). The deletion of the mG900 did not affect the development of lymphocytes in steady-state conditions or allergic airway inflammation induced by papain. However, mG900KO mice exhibited reduced allergic inflammation and Th2 differentiation in the HDM-induced allergic airway inflammation. The analysis of the chromatin conformation around Gata3 by circular chromosome conformation capture coupled to high-throughput sequencing (4C-seq) revealed that the mG900 region interacted with the transcription start site of Gata3 with an influencing chromatin conformation in Th2 cells. These findings indicate that the mG900 region plays a pivotal role in Th2 differentiation and thus enhances allergic airway inflammation.
Keywords: GATA3; ILC2; Th2 cell; asthma; enhancer.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Papi A., Brightling C., Pedersen S. E., Reddel H. K., Asthma. Lancet 391, 783–800 (2018). - PubMed
-
- Tindemans I., Serafini N., DiSanto J. P., Hendriks R. W., GATA-3 function in innate and adaptive immunity. Immunity 41, 191–206 (2014). - PubMed
-
- Nakayama T., et al. , Th2 cells in health and disease. Annu. Rev. Immunol. 35, 53–84 (2017). - PubMed
-
- Walker J. A., McKenzie A. N. J., TH2 cell development and function. Nat. Rev. Immunol. 18, 121–133 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

