Performing Suction Blister Skin Biopsies
- PMID: 38924322
- PMCID: PMC11210708
- DOI: 10.1002/cpz1.1073
Performing Suction Blister Skin Biopsies
Abstract
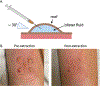
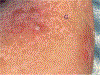
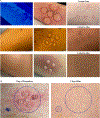
Traditional skin sampling methods include punch or shave biopsies to produce a solid tissue sample for analysis. These biopsy procedures are painful, require anesthesia, and leave permanent scars. This unit describes a suction blister skin biopsy method that can be used in place of traditional biopsy methodologies as a minimally invasive, non-scarring skin sampling technique. The induction of suction blisters uses an instrument with a chamber that applies negative pressure and gentle heat to the skin. Blister formation occurs within 1 hr, producing up to five blisters, each 10 mm in diameter per biopsy site. Blister fluid can be extracted and centrifuged to retrieve cells from the epidermis and upper dermis for flow cytometry, single-cell RNA sequencing, cell culture, and more without the need for digestion protocols. In addition, the blister fluid can be used to measure soluble proteins and metabolites. This unit describes the preparation of supplies and subjects, the suction blister biopsy procedure and blister formation, fluid extraction, and post-blistering care. © 2024 Wiley Periodicals LLC. Basic Protocol 1: Preparation of supplies and subject Basic Protocol 2: Suction blister biopsy procedure and formation Basic Protocol 3: Blister fluid extraction Basic Protocol 4: Post-blister care and clean up.
Keywords: biopsy; non‐scarring; skin; suction blister.
© 2024 Wiley Periodicals LLC.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT:
John Harris is a consultant for AbbVie Inc, Aldena, Almirall, Alys Pharmaceuticals, Avoro, Bain Capital, DrenBio, Granular Therapeutics Inc, Incyte, Klirna, Matchpoint Therapeutics, NIRA Biosciences, TeVido BioDevices, Vimela Therapeutics, and Vividion. John Harris is an investigator for Barinthus Bio NA, Cour Pharma, Incyte, NexImmune, and TeVido BioDevices. John Harris is scientific founder of Aldena, Alys Pharmaceuticals, Klirna, NIRA Biosciences and Vimela Therapeutics. John Harris has equity in Aldena, Alys Pharmaceuticals, Incyte, NIRA Biosciences, Rheos Medicines, TeVido BioDevices, and Vimela Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Dellatorre G, Bertolini W, & Castro CCSD. (2017). Optimizing suction blister epidermal graft technique in the surgical treatment of vitiligo. Anais Brasileiros de Dermatologia, 92, 888–890. https://www.scielo.br/j/abd/a/T5ZvWTTfPdLPG3J8SfTmZdz/ - PMC - PubMed
-
- Gellatly KJ, Strassner JP, Essien K, Refat MA, Murphy RL, Coffin-Schmitt A, Pandya AG, Tovar-Garza A, Frisoli ML, Fan X, Ding X, Kim EE, Abbas Z, McDonel P, Garber M, & Harris JE. (2021). scRNA-seq of human vitiligo reveals complex networks of subclinical immune activation and a role for CCR5 in Treg function. Science Translational Medicine, 13(610):eabd8995. doi: 10.1126/scitranslmed.abd8995. - DOI - PMC - PubMed
-
- Ingemansson-Nordqvist B, Kiistala U, & Rorsman H. (1967). Culture of adult human epidermal cells obtained from roofs of suction blisters. Acta dermato-venereologica, 47(4), 237–240.https://europepmc.org/article/med/4166058 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

