Lactate transported by MCT1 plays an active role in promoting mitochondrial biogenesis and enhancing TCA flux in skeletal muscle
- PMID: 38924407
- PMCID: PMC11204292
- DOI: 10.1126/sciadv.adn4508
Lactate transported by MCT1 plays an active role in promoting mitochondrial biogenesis and enhancing TCA flux in skeletal muscle
Abstract
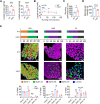
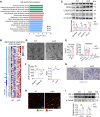
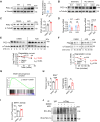
Once considered as a "metabolic waste," lactate is now recognized as a major fuel for tricarboxylic acid (TCA) cycle. Our metabolic flux analysis reveals that skeletal muscle mainly uses lactate to fuel TCA cycle. Lactate is transported through the cell membrane via monocarboxylate transporters (MCTs) in which MCT1 is highly expressed in the muscle. We analyzed how MCT1 affects muscle functions using mice with specific deletion of MCT1 in skeletal muscle. MCT1 deletion enhances running performance, increases oxidative fibers while decreasing glycolytic fibers, and enhances flux of glucose to TCA cycle. MCT1 deficiency increases the expression of mitochondrial proteins, augments cell respiration rate, and elevates mitochondrial activity in the muscle. Mechanistically, the protein level of PGC-1α, a master regulator of mitochondrial biogenesis, is elevated upon loss of MCT1 via increases in cellular NAD+ level and SIRT1 activity. Collectively, these results demonstrate that MCT1-mediated lactate shuttle plays a key role in regulating muscle functions by modulating mitochondrial biogenesis and TCA flux.
Figures





References
-
- Ferguson B. S., Rogatzki M. J., Goodwin M. L., Kane D. A., Rightmire Z., Gladden L. B., Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 118, 691–728 (2018). - PubMed
-
- Brooks G. A., The lactate shuttle during exercise and recovery. Med. Sci. Sports Exerc. 18, 360–368 (1986). - PubMed
-
- Brooks G. A., The science and translation of lactate shuttle theory. Cell Metab. 27, 757–785 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

