CMV reactivation during pretransplantation evaluation: a novel risk factor for posttransplantation CMV reactivation
- PMID: 38924728
- PMCID: PMC11399585
- DOI: 10.1182/bloodadvances.2023012234
CMV reactivation during pretransplantation evaluation: a novel risk factor for posttransplantation CMV reactivation
Abstract
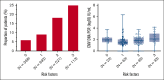
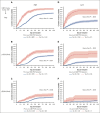
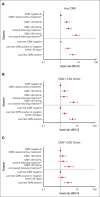
Cytomegalovirus (CMV) disease occurs occasionally before allogeneic hematopoietic cell transplantation (HCT) and is associated with poor post-HCT outcomes; however, the impact of pre-HCT CMV reactivation is unknown. Pre-HCT CMV reactivation was assessed in HCT candidates from the preemptive antiviral therapy (2007-2017) and letermovir prophylaxis (2018-2021) eras. CMV DNA polymerase chain reaction (PCR) surveillance was routinely performed during the pre-HCT workup period, and antiviral therapy was recommended according to risk of progression to CMV disease. Risk factors for pre-HCT CMV reactivation were characterized, and the associations of pre-HCT CMV reactivation with post-HCT outcomes were examined using logistic regression and Cox proportional hazard models, respectively. A total of 1694 patients were identified, and 11% had pre-HCT CMV reactivation 14 days (median; interquartile range [IQR], 6-23) before HCT. Lymphopenia (≤0.3 × 103/μL) was the strongest risk factor for pre-HCT CMV reactivation at multiple PCR levels. In the preemptive therapy era, patients with pre-HCT CMV reactivation had a significantly increased risk of CMV reactivation by day 100 as well as CMV disease and death by 1 year after HCT. Clearance of pre-HCT CMV reactivation was associated with a lower risk of post-HCT CMV reactivation. Similar associations with post-HCT CMV end points were observed in a cohort of patients receiving letermovir prophylaxis. Pre-HCT CMV reactivation can be routinely detected in high-risk HCT candidates and is a significant risk factor for post-HCT CMV reactivation and disease. Pre-HCT CMV DNA PCR surveillance is recommended in high-risk HCT candidates, and antiviral therapy may be indicated to prevent post-HCT CMV reactivation.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: L.H. reports research funding from Bristol Myers Squibb, Merck, Millennium-Takeda, and Sanofi; royalties from UpToDate; and has consulted for BioLineRX outside the submitted work. A.W. reports grants and personal fees from AlloVir, Ansun Biopharma, Kyorin Pharmaceutical, Pfizer, and Vir/GlaxoSmithKline outside the submitted work. A.L.G. reports central laboratory testing contracts from Abbott, Cepheid, Gilead, Hologic, Novavax, and Pfizer outside the submitted work. G.R.H. has consulted for GENERON Corporation, NapaJen Pharma, iTeos Therapeutics, Neoleukin Therapeutics, Commonwealth Serum Laboratories, and Cynata Therapeutics; and has received research funding from Compass Therapeutics, Syndax Pharmaceuticals, Applied Molecular Transport, SerPlus Technology, Heat Biologics, Laevoroc Oncology, iTeos Therapetics, and Genentech unrelated to the subject matter of this manuscript. J.A.H. reports research support from AlloVir, Merck, Oxford Immunotec, and Takeda; and personal fees from AlloVir, Moderna, Karius, and Gilead. M.J.B. reports personal fees from Merck, AlloVir, Symbio, Helocyte, Takeda, Moderna, and Evrys Bio outside the submitted work; and research support from Merck and Moderna. The remaining authors declare no competing financial interests.
Figures