Effect of 48 Months of Closed-Loop Insulin Delivery on Residual C-Peptide Secretion and Glycemic Control in Newly Diagnosed Youth With Type 1 Diabetes: A Randomized Trial
- PMID: 38924772
- PMCID: PMC11272979
- DOI: 10.2337/dc24-0360
Effect of 48 Months of Closed-Loop Insulin Delivery on Residual C-Peptide Secretion and Glycemic Control in Newly Diagnosed Youth With Type 1 Diabetes: A Randomized Trial
Abstract
Objective: We evaluated the effect of long-term intensive metabolic control with hybrid closed-loop (CL) on residual C-peptide secretion and glucose control compared with standard insulin therapy in youth with type 1 diabetes over 48 months.
Research design and methods: Following the 24-month primary phase of a multicenter, randomized, parallel trial of 96 newly diagnosed youth aged 10 to 16.9 years, participants were invited to an extension phase using treatment allocated at randomization. They continued with hybrid CL using the Cambridge algorithm or standard insulin therapy (control) until 48 months after diagnosis. Analysis was by intention-to-treat.
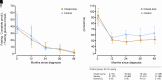
Results: At 24 months after diagnosis, 81 participants (mean ± SD age 14 ± 2 years) continued in the extension phase (47 CL, 34 control). There was no difference in fasting C-peptide corrected for fasting glucose at 48 months between groups (CL: 5 ± 9 vs. control: 6 ± 14 pmol/L per mmol/L; mean adjusted difference -2 [95% CI -7, 4; P = 0.54]). Central laboratory HbA1c remained lower in the CL group by 0.9% (10 mmol/mol [95% CI 0.2, 1.5; 3, 17 mmol/mol); P = 0.009). Time in target range of 3.9 to 10.0 mmol/L was 12 percentage points (95% CI 3, 20; P = 0.008) higher in the CL group compared with control. There were 11 severe hypoglycemic events (6 CL, 5 control) and 7 diabetic ketoacidosis events (3 CL, 4 control) during the extension phase.
Conclusions: Improved glycemic control was sustained over 48 months after diagnosis with CL insulin delivery compared with standard therapy in youth with type 1 diabetes. This did not appear to confer a protective effect on residual C-peptide secretion.
© 2024 by the American Diabetes Association.
Conflict of interest statement
Figures

References
-
- Todd JA. Etiology of type 1 diabetes. Immunity 2010;32:457–467 - PubMed
-
- Patterson CC, Karuranga S, Salpea P, et al. . Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107842. - PubMed
-
- Ward ZJ, Yeh JM, Reddy CL, et al. . Estimating the total incidence of type 1 diabetes in children and adolescents aged 0-19 years from 1990 to 2050: a global simulation-based analysis. Lancet Diabetes Endocrinol 2022;10:848–858 - PubMed
-
- National Paediatric Diabetes Audit . Annual report 2020-2021: care processes and outcomes. Royal College of Paediatrics and Child Health, 2022. Accessed 19 February 2024. Available from https://www.rcpch.ac.uk/sites/default/files/2022-04/National%20NPDA%20re...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

