Synthesis and biological characterization of an orally bioavailable lactate dehydrogenase-A inhibitor against pancreatic cancer
- PMID: 38925013
- PMCID: PMC12131164
- DOI: 10.1016/j.ejmech.2024.116598
Synthesis and biological characterization of an orally bioavailable lactate dehydrogenase-A inhibitor against pancreatic cancer
Abstract
Lactate dehydrogenase-A (LDHA) is the major isoform of lactate dehydrogenases (LDH) that is overexpressed and linked to poor survival in pancreatic ductal adenocarcinoma (PDAC). Despite some progress, current LDH inhibitors have poor structural and physicochemical properties or exhibit unfavorable pharmacokinetics that have hampered their development. The present study reports the synthesis and biological evaluation of a novel class of LDHA inhibitors comprising a succinic acid monoamide motif. Compounds 6 and 21 are structurally related analogs that demonstrated potent inhibition of LDHA with IC50s of 46 nM and 72 nM, respectively. We solved cocrystal structures of compound 21-bound to LDHA that showed that the compound binds to a distinct allosteric site between the two subunits of the LDHA tetramer. Inhibition of LDHA correlated with reduced lactate production and reduction of glycolysis in MIA PaCa-2 pancreatic cancer cells. The lead compounds inhibit the proliferation of human pancreatic cancer cell lines and patient-derived 3D organoids and exhibit a synergistic cytotoxic effect with the OXPHOS inhibitor phenformin. Unlike current LDHA inhibitors, 6 and 21 have appropriate pharmacokinetics and ligand efficiency metrics, exhibit up to 73% oral bioavailability, and a cumulative half-life greater than 4 h in mice.
Keywords: Lactate; Lactate Dehydrogenase-A; OXPHOS; Pancreatic cancer; Pharmacokinetics; Small molecule inhibitor.
Copyright © 2024 The Author(s). Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
Declaration of competing interest On behalf of all the authors, the corresponding author declares that there is no known financial interests or personal relationships that that could inappropriately influence (bias) the work reported in the manuscript.
Figures

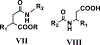












References
-
- Liberti MV, Locasale JW, The warburg effect: How does it benefit cancer cells? Trends Biochem. Sci 41 (3) (2016) 211–218, doi: S0968–0004(15)00241–8 [pii].
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

