KDM5A/B contribute to HIV-1 latent infection and survival of HIV-1 infected cells
- PMID: 38925368
- PMCID: PMC11927087
- DOI: 10.1016/j.antiviral.2024.105947
KDM5A/B contribute to HIV-1 latent infection and survival of HIV-1 infected cells
Abstract
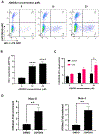
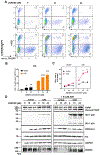
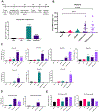
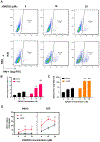
Combinational antiretroviral therapy (cART) suppresses human immunodeficiency virus type 1 (HIV-1) viral replication and pathogenesis in acquired immunodeficiency syndrome (AIDS) patients. However, HIV-1 remains in the latent stage of infection by suppressing viral transcription, which hinders an HIV-1 cure. One approach for an HIV-1 cure is the "shock and kill" strategy. The strategy focuses on reactivating latent HIV-1, inducing the viral cytopathic effect and facilitating the immune clearance for the elimination of latent HIV-1 reservoirs. Here, we reported that the H3K4 trimethylation (H3K4me3)-specific demethylase KDM5A/B play a role in suppressing HIV-1 Tat/LTR-mediated viral transcription in HIV-1 latent cells. Furthermore, we evaluated the potential of KDM5-specific inhibitor JQKD82 as an HIV-1 "shock and kill" agent. Our results showed that JQKD82 increases the H3K4me3 level at HIV-1 5' LTR promoter regions, HIV-1 reactivation, and the cytopathic effects in an HIV-1-latent T cell model. In addition, we identified that the combination of JQKD82 and AZD5582, a non-canonical NF-κB activator, generates a synergistic impact on inducing HIV-1 lytic reactivation and cell death in the T cell. The latency-reversing potency of the JQKD82 and AZD5582 pair was also confirmed in peripheral blood mononuclear cells (PBMCs) isolated from HIV-1 aviremic patients and in an HIV-1 latent monocyte. In latently infected microglia (HC69) of the brain, either deletion or inhibition of KDM5A/B results in a reversal of the HIV-1 latency. Overall, we concluded that KDM5A/B function as a host repressor of the HIV-1 lytic reactivation and thus promote the latency and the survival of HIV-1 infected reservoirs.
Keywords: AZD5582; CD4(+) T cells; Demethylation; Elimination; HIV-1; JQKD82; KDM5; Latency; Lytic reactivation; Microglia; Monocytes; Reservoir.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

