Comparison of Gout Flares With the Initiation of Treat-to-Target Allopurinol and Febuxostat: A Post-Hoc Analysis of a Randomized Multicenter Trial
- PMID: 38925627
- PMCID: PMC11421957
- DOI: 10.1002/art.42927
Comparison of Gout Flares With the Initiation of Treat-to-Target Allopurinol and Febuxostat: A Post-Hoc Analysis of a Randomized Multicenter Trial
Abstract
Objective: Initiating urate-lowering therapy (ULT) in gout can precipitate arthritis flares. There have been limited comparisons of flare risk during the initiation and escalation of allopurinol and febuxostat, administered as a treat-to-target strategy with optimal anti-inflammatory prophylaxis.
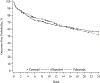
Methods: This was a post-hoc analysis of a 72-week randomized, double-blind, placebo-controlled, noninferiority trial comparing the efficacy of allopurinol and febuxostat. For this analysis, the occurrence of flares was examined during weeks 0 to 24 when ULT was initiated and titrated to a serum urate (sUA) goal of less than 6 mg/dl (<5 mg/dl if tophi). Flares were assessed at regular intervals through structured participant interviews. Predictors of flare, including treatment assignment, were examined using multivariable Cox proportional hazards regression.
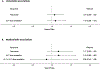
Results: Study participants (n = 940) were predominantly male (98.4%) and had a mean age of 62.1 years with approximately equal proportions receiving allopurinol or febuxostat. Mean baseline sUA was 8.5 mg/dl and all participants received anti-inflammatory prophylaxis (90% colchicine). In a multivariable model, there were no significant associations of ULT treatment (hazard ratio [HR] 1.17; febuxostat vs allopurinol), ULT-dose escalation (HR 1.18 vs no escalation), prophylaxis type, or individual comorbidity with flare and no evidence of ULT-dose escalation interaction. Factors independently associated with flare risk during ULT initiation/escalation included younger age, higher baseline sUA, and absence of tophi.
Conclusion: These results demonstrate that gout flare risk during the initiation and titration of allopurinol is similar to febuxostat when these agents are administered according to a treat-to-target strategy using gradual ULT-dose titration and best practice gout flare prophylaxis.
© 2024 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
TRM has served as a consultant for Horizon Therapeutics, Olatech Therapeutics, Pfizer, UCB, and Sanofi and receives research support from Horizon. BRE has received consulting fees and research support from Boehringer-Ingelheim. MHP has served as a consultant for Horizon Therapeutics, Sobi, Federation Bio, Fortress Bioscience and Scilex, and receives research support from Hikma Pharmaceuticals. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Figures
References
-
- Edwards NL, Sundy JS, Forsythe A, et al. Work productivity loss due to flares in patients with chronic gout refractory to conventional therapy. J Med Econ. 2011; 14(1):10–5. - PubMed
-
- Sigurdardottir V, Drivelegka P, Svärd A, et al. Work disability in gout: a population-based case–control study. Ann Rheum Dis. 2018; 77(3):399–404. - PubMed
-
- Becker MA, MacDonald PA, Hunt BJ, et al. Determinants of the clinical outcomes of gout during the first year of urate-lowering therapy. Nucleosides Nucleotides Nucleic Acids. 2008; 27(6):585–91. - PubMed
-
- Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005; 353(23):2450–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- I01 CX002358/CX/CSRD VA/United States
- UL1 TR001445/TR/NCATS NIH HHS/United States
- BX-00-4600/VA/VA/United States
- IK2 CX002203/CX/CSRD VA/United States
- 1UL1-TR-001445/GF/NIH HHS/United States
- I01-CX-002358/VA/VA/United States
- IK6 BX004600/BX/BLRD VA/United States
- IK2-CX-002203/VA CSR&D
- U54-GM-115458/GF/NIH HHS/United States
- P30-AR-072571/GF/NIH HHS/United States
- P30 AR072571/AR/NIAMS NIH HHS/United States
- I01 BX003635/BX/BLRD VA/United States
- VA Cooperative Studies Program of the Department of Veterans Affairs Office of Research & Development
- K24 AR070892/AR/NIAMS NIH HHS/United States
- K24-AR-070892/GF/NIH HHS/United States
- U54 GM115458/GM/NIGMS NIH HHS/United States
- Rheumatology Research Foundation
LinkOut - more resources
Full Text Sources
Medical

