Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: population based study
- PMID: 38925788
- PMCID: PMC11203392
- DOI: 10.1136/bmj-2023-078523
Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: population based study
Erratum in
-
Trends in cardiovascular disease incidence among 22 million people in the UK over 20 years: population based study.BMJ. 2024 Oct 28;387:q2381. doi: 10.1136/bmj.q2381. BMJ. 2024. PMID: 39467586 Free PMC article. No abstract available.
Abstract
Objective: To investigate the incidence of cardiovascular disease (CVD) overall and by age, sex, and socioeconomic status, and its variation over time, in the UK during 2000-19.
Design: Population based study.
Setting: UK.
Participants: 1 650 052 individuals registered with a general practice contributing to Clinical Practice Research Datalink and newly diagnosed with at least one CVD from 1 January 2000 to 30 June 2019.
Main outcome measures: The primary outcome was incident diagnosis of CVD, comprising acute coronary syndrome, aortic aneurysm, aortic stenosis, atrial fibrillation or flutter, chronic ischaemic heart disease, heart failure, peripheral artery disease, second or third degree heart block, stroke (ischaemic, haemorrhagic, and unspecified), and venous thromboembolism (deep vein thrombosis or pulmonary embolism). Disease incidence rates were calculated individually and as a composite outcome of all 10 CVDs combined and were standardised for age and sex using the 2013 European standard population. Negative binomial regression models investigated temporal trends and variation by age, sex, and socioeconomic status.
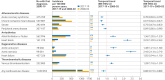
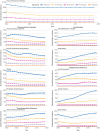
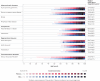
Results: The mean age of the population was 70.5 years and 47.6% (n=784 904) were women. The age and sex standardised incidence of all 10 prespecified CVDs declined by 19% during 2000-19 (incidence rate ratio 2017-19 v 2000-02: 0.80, 95% confidence interval 0.73 to 0.88). The incidence of coronary heart disease and stroke decreased by about 30% (incidence rate ratios for acute coronary syndrome, chronic ischaemic heart disease, and stroke were 0.70 (0.69 to 0.70), 0.67 (0.66 to 0.67), and 0.75 (0.67 to 0.83), respectively). In parallel, an increasing number of diagnoses of cardiac arrhythmias, valve disease, and thromboembolic diseases were observed. As a result, the overall incidence of CVDs across the 10 conditions remained relatively stable from the mid-2000s. Age stratified analyses further showed that the observed decline in coronary heart disease incidence was largely restricted to age groups older than 60 years, with little or no improvement in younger age groups. Trends were generally similar between men and women. A socioeconomic gradient was observed for almost every CVD investigated. The gradient did not decrease over time and was most noticeable for peripheral artery disease (incidence rate ratio most deprived v least deprived: 1.98 (1.87 to 2.09)), acute coronary syndrome (1.55 (1.54 to 1.57)), and heart failure (1.50 (1.41 to 1.59)).
Conclusions: Despite substantial improvements in the prevention of atherosclerotic diseases in the UK, the overall burden of CVDs remained high during 2000-19. For CVDs to decrease further, future prevention strategies might need to consider a broader spectrum of conditions, including arrhythmias, valve diseases, and thromboembolism, and examine the specific needs of younger age groups and socioeconomically deprived populations.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: NC is funded by a personal fellowship from the Research Foundation Flanders and a research grant from the European Society of Cardiology. JMF, PSJ, JGC, NS, and JJVM are supported by British Heart Foundation Centre of Research Excellence. PSJ and JJVM are further supported by the Vera Melrose Heart Failure Research Fund. JJVM has received funding to his institution from Amgen and Cytokinetics for his participation in the steering sommittee for the ATOMIC-HF, COSMIC-HF, and GALACTIC-HF trials and meetings and other activities related to these trials; has received payments through Glasgow University from work on clinical trials, consulting, and other activities from Alnylam, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Cardurion, Dal-Cor, GlaxoSmithKline, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos; and has received personal lecture fees from the Corpus, Abbott, Hikma, Sun Pharmaceuticals, Medscape/Heart.Org, Radcliffe Cardiology, Alkem Metabolics, Eris Lifesciences, Lupin, ProAdWise Communications, Servier Director, and Global Clinical Trial Partners. NS declares consulting fees or speaker honorariums, or both, from Abbott Laboratories, Afimmune, Amgen, AstraZeneca, Boehringer Ingelheim, Lilly, Hanmi Pharmaceuticals, Janssen, Merck Sharp & Dohme, Novartis, Novo Nordisk, Pfizer, Roche Diagnostics, and Sanofi; and grant support paid to his university from AstraZeneca, Boehringer Ingelheim, Novartis, and Roche Diagnostics. KK has acted as a consultant or speaker or received grants for investigator initiated studies for Astra Zeneca, Bayer, Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, Merck Sharp & Dohme, Boehringer Ingelheim, Oramed Pharmaceuticals, Roche, and Applied Therapeutics. KK is supported by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration East Midlands (ARC EM) and the NIHR Leicester Biomedical Research Centre (BRC). CL is funded by an NIHR Advanced Research Fellowship (NIHR-300111) and supported by the Leicester BRC. PSJ has received speaker fees from AstraZeneca, Novartis, Alkem Metabolics, ProAdWise Communications, Sun Pharmaceuticals, and Intas Pharmaceuticals; has received advisory board fees from AstraZeneca, Boehringer Ingelheim, and Novartis; has received research funding from AstraZeneca, Boehringer Ingelheim, Analog Devices; his employer, the University of Glasgow, has been remunerated for clinical trial work from AstraZeneca, Bayer, Novartis, and Novo Nordisk; and is the Director of Global Clinical Trial Partners. HS is supported by the China Scholarship Council. Other authors report no support from any organisation for the submitted work, no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Figures



References
-
- Institute for Health Metrics and Evaluation. Gobal Burden of Diseases Viz Hub. 2023. https://vizhub.healthdata.org/gbd-compare/
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
