Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial
- PMID: 38925803
- PMCID: PMC11200154
- DOI: 10.1136/bmj-2023-079061
Colchicine in patients with acute ischaemic stroke or transient ischaemic attack (CHANCE-3): multicentre, double blind, randomised, placebo controlled trial
Abstract
Objectives: To assess the efficacy and safety of colchicine versus placebo on reducing the risk of subsequent stroke after high risk non-cardioembolic ischaemic stroke or transient ischaemic attack within the first three months of symptom onset (CHANCE-3).
Design: Multicentre, double blind, randomised, placebo controlled trial.
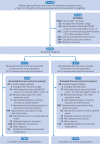
Setting: 244 hospitals in China between 11 August 2022 and 13 April 2023.
Participants: 8343 patients aged 40 years of age or older with a minor-to-moderate ischaemic stroke or transient ischaemic attack and a high sensitivity C-reactive protein ≥2 mg/L were enrolled.
Interventions: Patients were randomly assigned 1:1 within 24 h of symptom onset to receive colchicine (0.5 mg twice daily on days 1-3, followed by 0.5 mg daily thereafter) or placebo for 90 days.
Main outcome measures: The primary efficacy outcome was any new stroke within 90 days after randomisation. The primary safety outcome was any serious adverse event during the treatment period. All efficacy and safety analyses were by intention to treat.
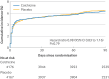
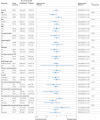
Results: 4176 patients were assigned to the colchicine group and 4167 were assigned to the placebo group. Stroke occurred within 90 days in 264 patients (6.3%) in the colchicine group and 270 patients (6.5%) in the placebo group (hazard ratio 0.98 (95% confidence interval 0.83 to 1.16); P=0.79). Any serious adverse event was observed in 91 (2.2%) patients in the colchicine group and 88 (2.1%) in the placebo group (P=0.83).
Conclusions: The study did not provide evidence that low-dose colchicine could reduce the risk of subsequent stroke within 90 days as compared with placebo among patients with acute non-cardioembolic minor-to-moderate ischaemic stroke or transient ischaemic attack and a high sensitivity C-reactive protein ≥2 mg/L.
Trial registration: ClinicalTrials.gov, NCT05439356.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Declaration of interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from National Key R&D Program of China, the National Natural Science Foundation of China, the Capital's Funds for Health Improvement and Research, and Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Pan Y, Meng X, Jin A, et al. . Time course for benefit and risk with ticagrelor and aspirin in individuals with acute ischemic stroke or transient ischemic attack who carry CYP2C19 loss-of-function alleles: a secondary analysis of the CHANCE-2 randomized clinical trial. JAMA Neurol 2022;79:739-45. 10.1001/jamaneurol.2022.1457. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
