Gut microbiota signatures of vulnerability to food addiction in mice and humans
- PMID: 38926079
- PMCID: PMC11503113
- DOI: 10.1136/gutjnl-2023-331445
Gut microbiota signatures of vulnerability to food addiction in mice and humans
Abstract
Objective: Food addiction is a multifactorial disorder characterised by a loss of control over food intake that may promote obesity and alter gut microbiota composition. We have investigated the potential involvement of the gut microbiota in the mechanisms underlying food addiction.
Design: We used the Yale Food Addiction Scale (YFAS) 2.0 criteria to classify extreme food addiction in mouse and human subpopulations to identify gut microbiota signatures associated with vulnerability to this disorder.
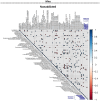
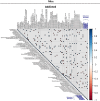
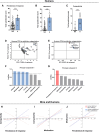
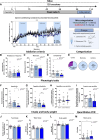
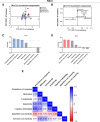
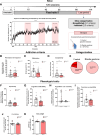
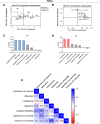
Results: Both animal and human cohorts showed important similarities in the gut microbiota signatures linked to food addiction. The signatures suggested possible non-beneficial effects of bacteria belonging to the Proteobacteria phylum and potential protective effects of Actinobacteria against the development of food addiction in both cohorts of humans and mice. A decreased relative abundance of the species Blautia wexlerae was observed in addicted humans and of Blautia genus in addicted mice. Administration of the non-digestible carbohydrates, lactulose and rhamnose, known to favour Blautia growth, led to increased relative abundance of Blautia in mice faeces in parallel with dramatic improvements in food addiction. A similar improvement was revealed after oral administration of Blautia wexlerae as a beneficial microbe.
Conclusion: By understanding the crosstalk between this behavioural alteration and gut microbiota, these findings constitute a step forward to future treatments for food addiction and related eating disorders.
Keywords: NEUROPHARMACOLOGY; NUTRITION; OBESITY; PSYCHOLOGY.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
