Protocol for the development of a core outcome set for clinical trials in primary sclerosing cholangitis
- PMID: 38926149
- PMCID: PMC11216047
- DOI: 10.1136/bmjopen-2023-080143
Protocol for the development of a core outcome set for clinical trials in primary sclerosing cholangitis
Abstract
Background: Primary sclerosing cholangitis (PSC) is a progressive immune-mediated liver disease, for which no medical therapy has been shown to slow disease progression. However, the horizon for new therapies is encouraging, with several innovative clinical trials in progress. Despite these advancements, there is considerable heterogeneity in the outcomes studied, with lack of consensus as to what outcomes to measure, when to measure and how to measure. Furthermore, there has been a paradigm shift in PSC treatment targets over recent years, moving from biochemistry-based endpoints to histological assessment of liver fibrosis, imaging-based biomarkers and patient-reported outcome measures. The abundance of new interventional trials and evolving endpoints pose opportunities for all stakeholders involved in evaluating novel therapies. To this effect, there is a need to harmonise measures used in clinical trials through the development of a core outcome set (COS).
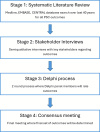
Methods and analysis: Synthesis of a PSC-specific COS will be conducted in four stages. Initially, a systematic literature review will be performed to identify outcomes previously used in PSC trials, followed by semistructured qualitative interviews conducted with key stakeholders. The latter may include patients, clinicians, researchers, pharmaceutical industry representatives and healthcare payers and regulatory agencies, to identify additional outcomes of importance. Using the outcomes generated from the literature review and stakeholder interviews, an international two-round Delphi survey will be conducted to prioritise outcomes for inclusion in the COS. Finally, a consensus meeting will be convened to ratify the COS and disseminate findings for application in future PSC trials.
Ethics and dissemination: Ethical approval has been granted by the East Midlands-Leicester Central Research Ethics Committee (Ref: 24/EM/0126) for this study. The COS from this study will be widely disseminated including publication in peer-reviewed journals, international conferences, promotion through patient-support groups and made available on the Core Outcomes Measurement in Effectiveness Trials (COMET) database.
Trial registration number: 1239.
Keywords: GASTROENTEROLOGY; Hepatobiliary disease; Hepatobiliary surgery; Hepatology; Inflammatory bowel disease; Patient Reported Outcome Measures.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: PJT has received grant support from the Wellcome Trust, the Medical Research Foundation, LifeArc, GSK, Guts UK, PSC Support, Intercept Pharma, Dr. Falk Pharma, Gilead sciences, Regeneron, and Bristol Myers Squibb. He has also received speaker fees from Albireo/IPSEN, Intercept and Dr Falk, and advisory board/consultancy fees from Cymabay, Intercept Pharma, Albireo/IPSEN, Pliant Pharma, Chemomab, Dr. Falk Pharma and GSK. OLA declares personal fees from Gilead Sciences, Merck and GlaxoSmithKline outside the submitted work and receives funding from the NIHR Birmingham Biomedical Research Centre (BRC), NIHR Applied Research Collaboration (ARC), West Midlands, NIHR Blood and Transplant Research Unit (BTRU) in Precision Transplant and Cellular Therapeutics at the University of Birmingham and University Hospitals Birmingham NHS Foundation, Innovate UK (part of UK Research and Innovation), Gilead Sciences Ltd, Merck, Anthony Nolan, and Sarcoma UK. MV has received speaker fees from Intercept, Lilly, Siemens Healthineers and GE Healthcare. CP received grant support from Gilead and Perspectum, consultancy fees from Pliant and Chemomab and speaker’s fees from Tillotts. MT received grant support from Albireo, Alnylam, Cymabay, Falk, Gilead, Intercept, MSD, Takeda and UltraGenyx; honoraria for consulting from Abbvie, Albireo, Boehringer Ingelheim, BiomX, Falk, Genfit, Gilead, Hightide, Intercept, Jannsen, MSD, Novartis, Phenex, Pliant, Regulus, Siemens and Shire; speaker fees from Albireo, Bristol-Myers Squibb, Falk, Gilead, Intercept, MSD and Madrigal, as well as travel support from AbbVie, Falk, Gilead and Intercept. He is also co-inventor on patents on the medical use of norUDCA/norucholic acid filed by the Medical University of Vienna. MW (on behalf of PSC Support) has received travel/speaker fees from CymaBay, Gilead Sciences, Dr Falk Pharma UK and Perspectum Diagnostics, and consultancy fees from GSK. The views expressed are those of the author(s) and not necessarily those of the National Health Service, the NIHR, or the Department of Health. Funders had no role in the design and conduct of the study, including preparation and review of the manuscript.