An ultrawide-range photochromic molecular fluorescence emitter
- PMID: 38926352
- PMCID: PMC11208420
- DOI: 10.1038/s41467-024-49670-7
An ultrawide-range photochromic molecular fluorescence emitter
Abstract
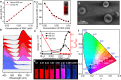
Photocontrollable luminescent molecular switches capable of changing emitting color have been regarded as the ideal integration between intelligent and luminescent materials. A remaining challenge is to combine good luminescence properties with wide range of wavelength transformation, especially when confined in a single molecular system that forms well-defined nanostructures. Here, we report a π-expanded photochromic molecular photoswitch, which allows for the comprehensive achievements including wide emission wavelength variation (240 nm wide, 400-640 nm), high photoisomerization extent (95%), and pure emission color (<100 nm of full width at half maximum). We take the advantageous mechanism of modulating self-assembly and intramolecular charge transfer in the synthesis and construction, and further realize the full color emission by simple photocontrol. Based on this, both photoactivated anti-counterfeiting function and self-erasing photowriting films are achieved of fluorescence. This work will provide insight into the design of intelligent optical materials.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Q. Li (Ed.) Intelligent stimuli responsive materials: from well-defined nanostructures to applications (John Wiley & Sons, Hoboken, NJ, 2013).
-
- Q. Li (Ed.) Photoactive functional soft materials: preparation, properties, and applications (Wiley-VCH, Weinheim, 2018).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

