Systemic prime mucosal boost significantly increases protective efficacy of bivalent RSV influenza viral vectored vaccine
- PMID: 38926455
- PMCID: PMC11208422
- DOI: 10.1038/s41541-024-00912-1
Systemic prime mucosal boost significantly increases protective efficacy of bivalent RSV influenza viral vectored vaccine
Abstract
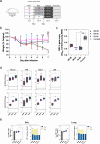
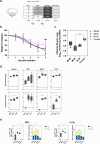
Although licensed vaccines against influenza virus have been successful in reducing pathogen-mediated disease, they have been less effective at preventing viral infection of the airways and current seasonal updates to influenza vaccines do not always successfully accommodate viral drift. Most licensed influenza and recently licensed RSV vaccines are administered via the intramuscular route. Alternative immunisation strategies, such as intranasal vaccinations, and "prime-pull" regimens, may deliver a more sterilising form of protection against respiratory viruses. A bivalent ChAdOx1-based vaccine (ChAdOx1-NP + M1-RSVF) encoding conserved nucleoprotein and matrix 1 proteins from influenza A virus and a modified pre-fusion stabilised RSV A F protein, was designed, developed and tested in preclinical animal models. The aim was to induce broad, cross-protective tissue-resident T cells against heterotypic influenza viruses and neutralising antibodies against RSV in the respiratory mucosa and systemically. When administered via an intramuscular prime-intranasal boost (IM-IN) regimen in mice, superior protection was generated against challenge with either RSV A, Influenza A H3N2 or H1N1. These results support further clinical development of a pan influenza & RSV vaccine administered in a prime-pull regimen.
© 2024. The Author(s).
Conflict of interest statement
The authors of this manuscript have the following competing interests: S.G. is the co-founder and a board member of Barinthus Biotherapeutics (formerly Vaccitech), and is named as an inventor on patents covering the use of ChAdOx1 vector vaccines. T.L. was a consultant to Barinthus Biotherapeutics on an unrelated project involving ChAdOx1 vector vaccines. She is also named as an inventor on a patent involving unrelated ChAdOx1 vector vaccines. T.L. also reports an honorarium from Seqirus. S.S. is senior director of research at Barinthus Biotherapeutics. All other authors declare no competing interests.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

