FRMD6 determines the cell fate towards senescence: involvement of the Hippo-YAP-CCN3 axis
- PMID: 38926528
- PMCID: PMC11519602
- DOI: 10.1038/s41418-024-01333-2
FRMD6 determines the cell fate towards senescence: involvement of the Hippo-YAP-CCN3 axis
Abstract
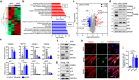
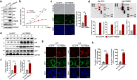
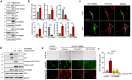
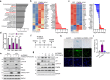
Cellular senescence, a hallmark of aging, is pathogenically linked to the development of aging-related diseases. This study demonstrates that FRMD6, an upstream component of the Hippo/YAP signaling cascade, is a key regulator of senescence. Proteomic analysis revealed that FRMD6 is upregulated in senescent IMR90 fibroblasts under various senescence-inducing conditions. Silencing FRMD6 mitigated the senescence of IMR90 cells, suggesting its requirement in senescence. Conversely, the overexpression of FRMD6 alone induced senescence in cells and in lung tissue, establishing a causal link. The elevated FRMD6 levels correlated well with increased levels of the inhibitory phosphorylated YAP/TAZ. We identified cellular communication network factor 3 (CCN3), a key component of the senescence-associated secretory phenotype regulated by YAP, whose administration attenuated FRMD6-induced senescence in a dose-dependent manner. Mechanistically, FRMD6 interacted with and activated MST kinase, which led to YAP/TAZ inactivation. The expression of FRMD6 was regulated by the p53 and SMAD transcription factors in senescent cells. Accordingly, the expression of FRMD6 was upregulated by TGF-β treatment that activates those transcription factors. In TGF-β-treated IMR90 cells, FRMD6 mainly segregated with p21, a senescence marker, but rarely segregated with α-SMA, a myofibroblast marker, which suggests that FRMD6 has a role in directing cells towards senescence. Similarly, in TGF-β-enriched environments, such as fibroblastic foci (FF) from patients with idiopathic pulmonary fibrosis, FRMD6 co-localized with p16 in FF lining cells, while it was rarely detected in α-SMA-positive myofibroblasts that are abundant in FF. In sum, this study identifies FRMD6 as a novel regulator of senescence and elucidates the contribution of the FRMD6-Hippo/YAP-CCN3 axis to senescence.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Barnes PJ, Baker J, Donnelly LE. Cellular senescence as a mechanism and target in chronic lung diseases. Am J Respir Crit Care Med. 2019;200:556–64. - PubMed
-
- Edgar BA. From cell structure to transcription: hippo forges a new path. Cell. 2006;124:267–73. - PubMed
-
- Zeng Q, Hong W. The emerging role of the hippo pathway in cell contact inhibition, organ size control, and cancer development in mammals. Cancer Cell. 2008;13:188–92. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

