Tracing the invisible mutant ADNP protein in Helsmoortel-Van der Aa syndrome patients
- PMID: 38926592
- PMCID: PMC11208605
- DOI: 10.1038/s41598-024-65608-x
Tracing the invisible mutant ADNP protein in Helsmoortel-Van der Aa syndrome patients
Abstract
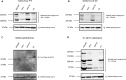
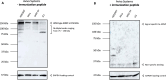
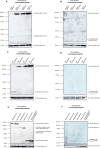
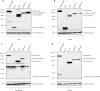
Heterozygous de novo mutations in the Activity-Dependent Neuroprotective Homeobox (ADNP) gene underlie Helsmoortel-Van der Aa syndrome (HVDAS). Most of these mutations are situated in the last exon and we previously demonstrated escape from nonsense-mediated decay by detecting mutant ADNP mRNA in patient blood. In this study, wild-type and ADNP mutants are investigated at the protein level and therefore optimal detection of the protein is required. Detection of ADNP by means of western blotting has been ambiguous with reported antibodies resulting in non-specific bands without unique ADNP signal. Validation of an N-terminal ADNP antibody (Aviva Systems) using a blocking peptide competition assay allowed to differentiate between specific and non-specific signals in different sample materials, resulting in a unique band signal around 150 kDa for ADNP, above its theoretical molecular weight of 124 kDa. Detection with different C-terminal antibodies confirmed the signals at an observed molecular weight of 150 kDa. Our antibody panel was subsequently tested by immunoblotting, comparing parental and homozygous CRISPR/Cas9 endonuclease-mediated Adnp knockout cell lines and showed disappearance of the 150 kDa signal, indicative for intact ADNP. By means of both a GFPSpark and Flag-tag N-terminally fused to a human ADNP expression vector, we detected wild-type ADNP together with mutant forms after introduction of patient mutations in E. coli expression systems by site-directed mutagenesis. Furthermore, we were also able to visualize endogenous ADNP with our C-terminal antibody panel in heterozygous cell lines carrying ADNP patient mutations, while the truncated ADNP mutants could only be detected with epitope-tag-specific antibodies, suggesting that addition of an epitope-tag possibly helps stabilizing the protein. However, western blotting of patient-derived hiPSCs, immortalized lymphoblastoid cell lines and post-mortem patient brain material failed to detect a native mutant ADNP protein. In addition, an N-terminal immunoprecipitation-competent ADNP antibody enriched truncating mutants in overexpression lysates, whereas implementation of the same method failed to enrich a possible native mutant protein in immortalized patient-derived lymphoblastoid cell lines. This study aims to shape awareness for critical assessment of mutant ADNP protein analysis in Helsmoortel-Van der Aa syndrome.
Keywords: Adnp knock-out cell line; Activity-dependent neuroprotective protein (ADNP); Antibody validation; Blocking peptide competition assay; Helsmoortel-Van der Aa syndrome (HVDAS); Immunoprecipitation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

