VTA glutamatergic projections to the nucleus accumbens suppress psychostimulant-seeking behavior
- PMID: 38926603
- PMCID: PMC11473768
- DOI: 10.1038/s41386-024-01905-3
VTA glutamatergic projections to the nucleus accumbens suppress psychostimulant-seeking behavior
Abstract
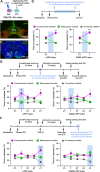
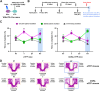
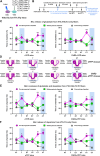
Converging evidence indicates that both dopamine and glutamate neurotransmission within the nucleus accumbens (NAc) play a role in psychostimulant self-administration and relapse in rodent models. Increased NAc dopamine release from ventral tegmental area (VTA) inputs is critical to psychostimulant self-administration and NAc glutamate release from prelimbic prefrontal cortex (PFC) inputs synapsing on medium spiny neurons (MSNs) is critical to reinstatement of psychostimulant-seeking after extinction. The regulation of the activity of MSNs by VTA dopamine inputs has been extensively studied, and recent findings have demonstrated that VTA glutamate neurons target the NAc medial shell. Here, we determined whether the mesoaccumbal glutamatergic pathway plays a role in psychostimulant conditioned place preference and self-administration in mice. We used optogenetics to induce NAc release of glutamate from VTA inputs during the acquisition, expression, and reinstatement phases of cocaine- or methamphetamine-induced conditioned place preference (CPP), and during priming-induced reinstatement of cocaine-seeking behavior. We found that NAc medial shell release of glutamate resulting from the activation of VTA glutamatergic fibers did not affect the acquisition of cocaine-induced CPP, but it blocked the expression, stress- and priming-induced reinstatement of cocaine- and methamphetamine CPP, as well as it blocked the priming-induced reinstatement of cocaine-seeking behavior after extinction. These findings indicate that in contrast to the well-recognized mesoaccumbal dopamine system that is critical to psychostimulant reward and relapse, there is a parallel mesoaccumbal glutamatergic system that suppresses reward and psychostimulant-seeking behavior.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–91. - PubMed
-
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–94. - PubMed
-
- Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharm Rev. 2002;54:1–42. - PubMed
-
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharm Biochem Behav. 1982;17:901–4. - PubMed
-
- Morales M, Barbano MF. Midbrain (VTA) circuits. In: Gilpin NW, editor. Neurocircuitry of addiction. Cambridge: Elsevier; 2023, pp 45–72.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

