9-cis-retinoic acid signaling in Sertoli cells regulates their immunomodulatory function to control lymphocyte physiology and Treg differentiation
- PMID: 38926848
- PMCID: PMC11202360
- DOI: 10.1186/s12958-024-01246-2
9-cis-retinoic acid signaling in Sertoli cells regulates their immunomodulatory function to control lymphocyte physiology and Treg differentiation
Abstract
Background: Testis is an immune privileged organ, which prevents the immune response against sperm antigens and inflammation. Testicular cells responsible for immune tolerance are mainly Sertoli cells, which form the blood-testis barrier and produce immunosuppressive factors. Sertoli cells prevent inflammation in the testis and maintain immune tolerance by inhibiting proliferation and inducing lymphocyte apoptosis. It has been shown that 9-cis-retinoic acid (9cRA) blocks ex vivo apoptosis of peripheral blood lymphocytes and promotes the differentiation of Treg cells in the gut. However, the role of retinoid signaling in regulating the immune privilege of the testes remains unknown.
Objective: The aim of this study was to determine whether 9cRA, acting via the retinoic acid receptors (RAR) and the retinoic X receptors (RXR), controls the immunomodulatory functions of Sertoli cells by influencing the secretion of anti-inflammatory/pro-inflammatory factors, lymphocyte physiology and Treg cell differentiation.
Methods: Experiments were performed using in vitro model of co-cultures of murine Sertoli cells and T lymphocytes. Agonists and antagonists of retinoic acid receptors were used to inhibit/stimulate retinoid signaling in Sertoli cells.
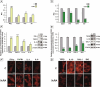
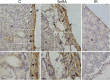
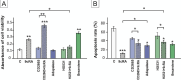
Results: Our results have demonstrated that 9cRA inhibits the expression of immunosuppressive genes and enhances the expression of pro-inflammatory factors in Sertoli cells and lymphocytes, increases lymphocyte viability and decreases apoptosis rate. Moreover, we have found that 9cRA blocks lymphocyte apoptosis acting through both RAR and RXR and inhibiting FasL/Fas/Caspase 8 and Bax/Bcl-2/Caspase 9 pathways. Finally, we have shown that 9cRA signaling in Sertoli cells inhibits Treg differentiation.
Conclusion: Collectively, our results indicate that retinoid signaling negatively regulates immunologically privileged functions of Sertoli cells, crucial for ensuring male fertility. 9cRA inhibits lymphocyte apoptosis, which can be related to the development of autoimmunity, inflammation, and, in consequence, infertility.
Keywords: 9-cis-retinoic acid; Sertoli cells; T lymphocyte apoptosis; Testis immune privilege; Treg differentiation.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
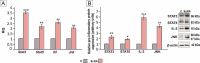



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

