Transcranial alternating current stimulation (tACS) at gamma frequency: an up-and-coming tool to modify the progression of Alzheimer's Disease
- PMID: 38926897
- PMCID: PMC11210106
- DOI: 10.1186/s40035-024-00423-y
Transcranial alternating current stimulation (tACS) at gamma frequency: an up-and-coming tool to modify the progression of Alzheimer's Disease
Abstract
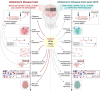
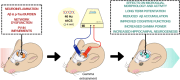
The last decades have witnessed huge efforts devoted to deciphering the pathological mechanisms underlying Alzheimer's Disease (AD) and to testing new drugs, with the recent FDA approval of two anti-amyloid monoclonal antibodies for AD treatment. Beyond these drug-based experimentations, a number of pre-clinical and clinical trials are exploring the benefits of alternative treatments, such as non-invasive stimulation techniques on AD neuropathology and symptoms. Among the different non-invasive brain stimulation approaches, transcranial alternating current stimulation (tACS) is gaining particular attention due to its ability to externally control gamma oscillations. Here, we outline the current knowledge concerning the clinical efficacy, safety, ease-of-use and cost-effectiveness of tACS on early and advanced AD, applied specifically at 40 Hz frequency, and also summarise pre-clinical results on validated models of AD and ongoing patient-centred trials.
Keywords: Alzheimer’s disease; Brain waves; Early intervention; Gamma oscillations; Mild cognitive impairment; Network dysfunction; Neuromodulation; Non-invasive brain stimulation; Parvalbumin interneurons; Theranostic approach.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Gauthier S. World Alzheimer Report 2022 – Life after diagnosis: Navigating treatment, care and support. London: Alzheimer’s Disease International; 2022.
-
- Eikelboom WS, van den Berg E, Singleton EH, Baart SJ, Coesmans M, Leeuwis AE, et al. Neuropsychiatric and Cognitive Symptoms Across the Alzheimer Disease Clinical Spectrum: Cross-sectional and Longitudinal Associations. Neurology. 2021;97:e1276–e1287. doi: 10.1212/WNL.0000000000012598. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

