Phase variable colony variants are conserved across Gardnerella spp. and exhibit different virulence-associated phenotypes
- PMID: 38926904
- PMCID: PMC11287997
- DOI: 10.1128/msphere.00450-24
Phase variable colony variants are conserved across Gardnerella spp. and exhibit different virulence-associated phenotypes
Abstract
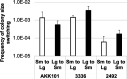
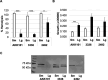
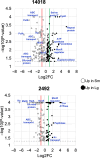
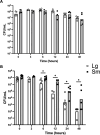
The Gardnerella genus, comprising at least 13 species, is associated with the polymicrobial disorder bacterial vaginosis (BV). However, the details of BV pathogenesis are poorly defined, and the contributions made by individual species, including Gardnerella spp., are largely unknown. We report here that colony phenotypes characterized by size (large and small) and opacity (opaque and translucent) are phase variable and are conserved among all tested Gardnerella strains, representing at least 10 different species. With the hypothesis that these different variants could be an important missing piece to the enigma of how BV develops in vivo, we characterized their phenotypic, proteomic, and genomic differences. Beyond increased colony size, large colony variants showed reduced vaginolysin secretion and faster growth rate relative to small colony variants. The ability to inhibit the growth of Neisseria gonorrhoeae and commensal Lactobacillus species varied by strain and, in some instances, differed between variants. Proteomics analyses indicated that 127-173 proteins were differentially expressed between variants. Proteins with increased expression in large variants of both strains were associated with amino acid and protein synthesis and protein folding, whereas those increased in small variants were related to nucleotide synthesis, phosphate transport, ABC transport, and glycogen breakdown. Furthermore, whole genome sequencing analyses revealed an abundance of genes associated with variable homopolymer tracts, implicating slipped strand mispairing in Gardnerella phase variation and illuminating the potential for previously unrecognized heterogeneity within clonal populations. Collectively, these results suggest that phase variants may be primed to serve different roles in BV pathogenesis.IMPORTANCEBacterial vaginosis is the most common gynecological disorder in women of childbearing age. Gardnerella species are crucial to the development of this dysbiosis, but the mechanisms involved in the infection are not understood. We discovered that Gardnerella species vary between two different forms, reflected in bacterial colony size. A slow-growing form makes large amounts of the toxin vaginolysin and is better able to survive in human cervix tissue. A fast-growing form is likely the one that proliferates to high numbers just prior to symptom onset and forms the biofilm that serves as a scaffold for multiple BV-associated anaerobic bacteria. Identification of the proteins that vary between different forms of the bacteria as well as those that vary randomly provides insight into the factors important for Gardnerella infection and immune avoidance.
Keywords: Gardnerella; phase variation; phenotypic variation; vaginosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Zevin AS, Xie IY, Birse K, Arnold K, Romas L, Westmacott G, Novak RM, McCorrister S, McKinnon LR, Cohen CR, Mackelprang R, Lingappa J, Lauffenburger DA, Klatt NR, Burgener AD. 2016. Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog 12:e1005889. doi: 10.1371/journal.ppat.1005889 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- T32AI055397/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21AI153534/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01AI113287/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI113287/AI/NIAID NIH HHS/United States
- T32 AI055397/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
