Activation of Peroxisome Proliferator-Activated Receptor-β/δ (PPARβ/δ) in Keratinocytes by Endogenous Fatty Acids
- PMID: 38927010
- PMCID: PMC11201440
- DOI: 10.3390/biom14060606
Activation of Peroxisome Proliferator-Activated Receptor-β/δ (PPARβ/δ) in Keratinocytes by Endogenous Fatty Acids
Abstract
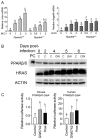
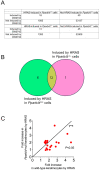
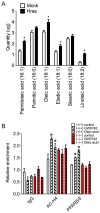
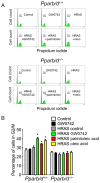
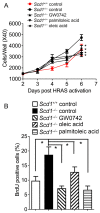
Nuclear hormone receptors exist in dynamic equilibrium between transcriptionally active and inactive complexes dependent on interactions with ligands, proteins, and chromatin. The present studies examined the hypothesis that endogenous ligands activate peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in keratinocytes. The phorbol ester treatment or HRAS infection of primary keratinocytes increased fatty acids that were associated with enhanced PPARβ/δ activity. Fatty acids caused PPARβ/δ-dependent increases in chromatin occupancy and the expression of angiopoietin-like protein 4 (Angptl4) mRNA. Analyses demonstrated that stearoyl Co-A desaturase 1 (Scd1) mediates an increase in intracellular monounsaturated fatty acids in keratinocytes that act as PPARβ/δ ligands. The activation of PPARβ/δ with palmitoleic or oleic acid causes arrest at the G2/M phase of the cell cycle of HRAS-expressing keratinocytes that is not found in similarly treated HRAS-expressing Pparb/d-null keratinocytes. HRAS-expressing Scd1-null mouse keratinocytes exhibit enhanced cell proliferation, an effect that is mitigated by treatment with palmitoleic or oleic acid. Consistent with these findings, the ligand activation of PPARβ/δ with GW0742 or oleic acid prevented UVB-induced non-melanoma skin carcinogenesis, an effect that required PPARβ/δ. The results from these studies demonstrate that PPARβ/δ has endogenous roles in keratinocytes and can be activated by lipids found in diet and cellular components.
Keywords: UVB-induced non-melanoma skin cancer; cell cycle; fatty acids; ligands; peroxisome proliferator-activated receptor.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

