Primary Nucleation of Polymorphic α-Synuclein Dimers Depends on Copper Concentrations and Definite Copper-Binding Site
- PMID: 38927031
- PMCID: PMC11201572
- DOI: 10.3390/biom14060627
Primary Nucleation of Polymorphic α-Synuclein Dimers Depends on Copper Concentrations and Definite Copper-Binding Site
Abstract
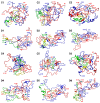
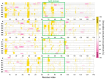
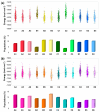
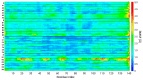
The primary nucleation process of α-synuclein (AS) that forms toxic oligomeric species is the early stage of the pathological cause of Parkinson's disease. It is well-known that copper influences this primary nucleation process. While significant efforts have been made to solve the structures of polymorphic AS fibrils, the structures of AS oligomers and the copper-bound AS oligomers at the molecular level and the effect of copper concentrations on the primary nucleation are elusive. Here, we propose and demonstrate new molecular mechanism pathways of primary nucleation of AS that are tuned by distinct copper concentrations and by a specific copper-binding site. We present the polymorphic AS dimers bound to different copper-binding sites at the atomic resolution in high- and low-copper concentrations, using extensive molecular dynamics simulations. Our results show the complexity of the primary nucleation pathways that rely on the copper concentrations and the copper binding site. From a broader perspective, our study proposes a new strategy to control the primary nucleation of other toxic amyloid oligomers in other neurodegenerative diseases.
Keywords: Parkinson’s disease; amyloids; metal ions; neurodegenerative diseases; oligomers; polymorphism; protein aggregation; self-assembly; α-synuclein.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





References
-
- Camino J.D., Gracia P., Chen S.W., Sot J., de la Arada I., Sebastian V., Arrondo J.L.R., Goni F.M., Dobson C.M., Cremades N. The extent of protein hydration dictates the preference for heterogeneous or homogeneous nucleation generating either parallel or antiparallel beta-sheet alpha-synuclein aggregates. Chem. Sci. 2020;11:11902–11914. doi: 10.1039/d0sc05297c. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

