Mitochondrial Reactive Oxygen Species in Infection and Immunity
- PMID: 38927073
- PMCID: PMC11202257
- DOI: 10.3390/biom14060670
Mitochondrial Reactive Oxygen Species in Infection and Immunity
Abstract
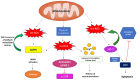
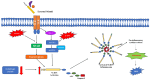
Reactive oxygen species (ROS) contain at least one oxygen atom and one or more unpaired electrons and include singlet oxygen, superoxide anion radical, hydroxyl radical, hydroperoxyl radical, and free nitrogen radicals. Intracellular ROS can be formed as a consequence of several factors, including ultra-violet (UV) radiation, electron leakage during aerobic respiration, inflammatory responses mediated by macrophages, and other external stimuli or stress. The enhanced production of ROS is termed oxidative stress and this leads to cellular damage, such as protein carbonylation, lipid peroxidation, deoxyribonucleic acid (DNA) damage, and base modifications. This damage may manifest in various pathological states, including ageing, cancer, neurological diseases, and metabolic disorders like diabetes. On the other hand, the optimum levels of ROS have been implicated in the regulation of many important physiological processes. For example, the ROS generated in the mitochondria (mitochondrial ROS or mt-ROS), as a byproduct of the electron transport chain (ETC), participate in a plethora of physiological functions, which include ageing, cell growth, cell proliferation, and immune response and regulation. In this current review, we will focus on the mechanisms by which mt-ROS regulate different pathways of host immune responses in the context of infection by bacteria, protozoan parasites, viruses, and fungi. We will also discuss how these pathogens, in turn, modulate mt-ROS to evade host immunity. We will conclude by briefly giving an overview of the potential therapeutic approaches involving mt-ROS in infectious diseases.
Keywords: bacteria; electron transport chain; fungi; inflammasome; mitochondrial reactive oxygen species; protozoa; virus.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

