Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model
- PMID: 38927075
- PMCID: PMC11201709
- DOI: 10.3390/biom14060672
Anti-Inflammatory Responses Produced with Nippostrongylus brasiliensis-Derived Uridine via the Mitochondrial ATP-Sensitive Potassium Channel and Its Anti-Atherosclerosis Effect in an Apolipoprotein E Gene Knockout Mouse Model
Abstract
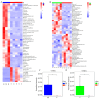
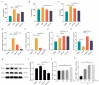
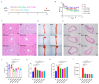
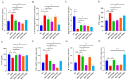
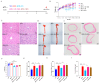
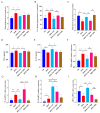
Atherosclerosis (AS) has become the leading cause of cardiovascular disease worldwide. Our previous study had observed that Nippostrongylus brasiliensis (Nb) infection or its derived products could inhibit AS development by inducing an anti-inflammatory response. We performed a metabolic analysis to screen Nb-derived metabolites with anti-inflammation activity and evaluated the AS-prevention effect. We observed that the metabolite uridine had higher expression levels in mice infected with the Nb and ES (excretory-secretory) products and could be selected as a key metabolite. ES and uridine interventions could reduce the pro-inflammatory responses and increase the anti-inflammatory responses in vitro and in vivo. The apolipoprotein E gene knockout (ApoE-/-) mice were fed with a high-fat diet for the AS modeling. Following the in vivo intervention, ES products or uridine significantly reduced serum and liver lipid levels, alleviated the formation of atherosclerosis, and reduced the pro-inflammatory responses in serum or plaques, while the anti-inflammatory responses showed opposite trends. After blocking with 5-HD (5-hydroxydecanoate sodium) in vitro, the mRNA levels of M2 markers were significantly reduced. When blocked with 5-HD in vivo, the degree of atherosclerosis was worsened, the pro-inflammatory responses were increased compared to the uridine group, while the anti-inflammatory responses decreased accordingly. Uridine, a key metabolite from Nippostrongylus brasiliensis, showed anti-inflammatory and anti-atherosclerotic effects in vitro and in vivo, which depend on the activation of the mitochondrial ATP-sensitive potassium channel.
Keywords: Nippostrongylus brasiliensis; anti-atherosclerosis; mitochondrial ATP-sensitive potassium channel; uridine.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Protective effects of Nippostrongylus brasiliensis-derived uridine via the apical sodium-dependent bile acid transporter in a mouse model of TNBS-induced inflammatory bowel disease.Front Immunol. 2025 May 5;16:1600838. doi: 10.3389/fimmu.2025.1600838. eCollection 2025. Front Immunol. 2025. PMID: 40391223 Free PMC article.
-
Inhibition Effects of Nippostrongylus brasiliensis and Its Derivatives against Atherosclerosis in ApoE-/- Mice through Anti-Inflammatory Response.Pathogens. 2022 Oct 20;11(10):1208. doi: 10.3390/pathogens11101208. Pathogens. 2022. PMID: 36297265 Free PMC article.
-
Mining Anti-Inflammation Molecules From Nippostrongylus brasiliensis-Derived Products Through the Metabolomics Approach.Front Cell Infect Microbiol. 2021 Nov 11;11:781132. doi: 10.3389/fcimb.2021.781132. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34858883 Free PMC article.
-
Copper chelation by tetrathiomolybdate inhibits vascular inflammation and atherosclerotic lesion development in apolipoprotein E-deficient mice.Atherosclerosis. 2012 Aug;223(2):306-13. doi: 10.1016/j.atherosclerosis.2012.06.013. Epub 2012 Jun 16. Atherosclerosis. 2012. PMID: 22770994 Free PMC article.
-
The role of an experimental model of atherosclerosis: apoE-knockout mice in developing new drugs against atherogenesis.Curr Pharm Biotechnol. 2012 Oct;13(13):2435-9. Curr Pharm Biotechnol. 2012. PMID: 22280417 Review.
Cited by
-
Protective effects of Nippostrongylus brasiliensis-derived uridine via the apical sodium-dependent bile acid transporter in a mouse model of TNBS-induced inflammatory bowel disease.Front Immunol. 2025 May 5;16:1600838. doi: 10.3389/fimmu.2025.1600838. eCollection 2025. Front Immunol. 2025. PMID: 40391223 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

